Figures & data
Scheme 1. The enzymatic reaction of Tnase with tryptophan results in pyruvate, ammonia and indole production [Citation8].
![Scheme 1. The enzymatic reaction of Tnase with tryptophan results in pyruvate, ammonia and indole production [Citation8].](/cms/asset/4a1edcc3-146f-48b7-b8c6-bb65a7b70db0/ienz_a_318928_f0001_b.gif)
Scheme 2. The α,β-elimination reaction of SOPC results in the production of o-nitrothiophenolate, pyruvate and ammonia.
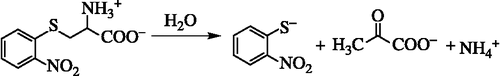
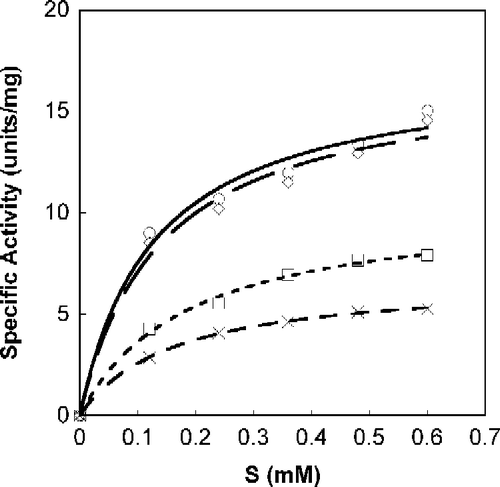
Figure 1. Michaelis-Menten plot of Tnase with S-phenyl-benzoquinone tryptophan. SOPC at different concentrations was used as a substrate, as described in the experimental section. Analyses were done with Calidagraph version 4. Measurements were performed in the absence, ○, and the presence of the inhibitor at a concentration of 47.5 μM, ◊; 95 μM, ▪; 190 μM, X.
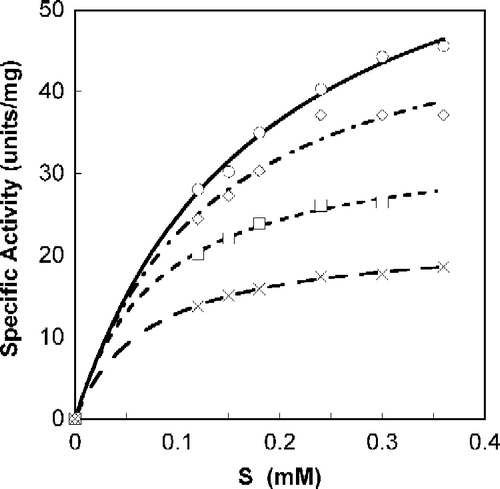
Table I. Kinetics parameters of the Tnase inhibitors.
Figure 2. Michaelis-Menten plot of Tnase with N-acetyl-tryptophan. SOPC was used as a substrate at different concentrations. Measurements were performed in the absence, ○, and the presence of the inhibitor at a concentration of 15.5 μM, ◊; 31 μM, ▪; 62 μM, X.
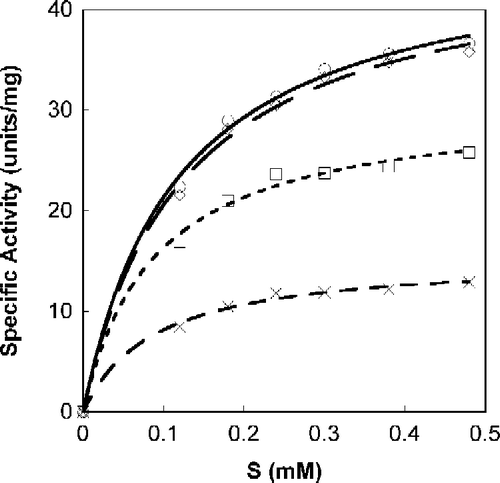
Figure 3. Michaelis-Menten plot of Tnase with p-Toluene sulfonic acid salt of L-tryptophan-ethylester. SOPC was used as a substrate at different concentrations. Measurements were performed in the absence, ○, and the presence of the inhibitor at a concentration of 26 μM, ◊; 52 μM, ▪.
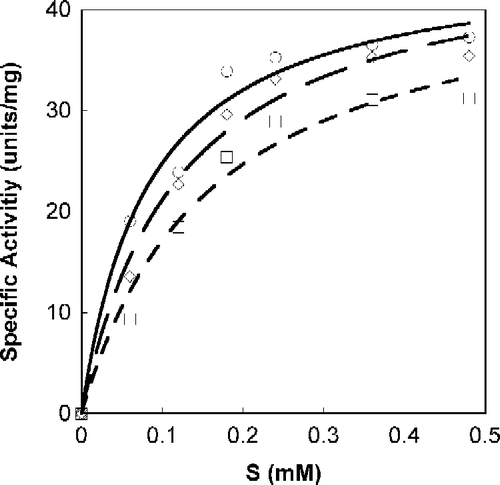
Figure 4. Michaelis-Menten plot of Tnase with α-Amino-2-(9,10-anthraquinone)-propanoic acid. SOPC was used as a substrate at different concentrations. Measurements were performed in the absence, ○, and the presence of the inhibitor at a concentration of 55 μM, ◊; 110 μM, ▪; 220 μM, X.
Scheme 3. The mechanism of catalysis of Tnase as proposed by Snell & Mari [Citation21].
![Scheme 3. The mechanism of catalysis of Tnase as proposed by Snell & Mari [Citation21].](/cms/asset/6d84e86d-994d-4a61-87a5-a3d958d6f3a5/ienz_a_318928_f0007_b.gif)