Figures & data
Table I. Structure and therapeutic application of retinoic acid isomers used clinically.
Figure 3. Diagram showing both enantiomers R and S of 4-{[4-(benzoxazol-2-ylamino)phenyl]imidazol-1-yl-methyl}benzoic acid docked at the active site region of the CYP26A1 model. Hydrogen bonding interactions are shown as green lines and co-ordination with the transition metal is indicated with a purple line. Colour coding of the atoms: grey = C, red = O, blue = N, brown = Fe and yellow = S. Amino-acid residues identified are involved in hydrophobic interactions.
![Figure 3. Diagram showing both enantiomers R and S of 4-{[4-(benzoxazol-2-ylamino)phenyl]imidazol-1-yl-methyl}benzoic acid docked at the active site region of the CYP26A1 model. Hydrogen bonding interactions are shown as green lines and co-ordination with the transition metal is indicated with a purple line. Colour coding of the atoms: grey = C, red = O, blue = N, brown = Fe and yellow = S. Amino-acid residues identified are involved in hydrophobic interactions.](/cms/asset/4662d6ef-d963-49bd-9b1e-c109aadc28a4/ienz_a_322000_f0003_b.gif)
Scheme 1. Reagents and Conditions: (i) NaBH4, MeOH, 1 h, rt, 60% (ii) H2, Pd/C, EtOH, 1 h, 48% (iii) CSCl2, CH2Cl2, H2O, 18 h, 0°C, 60–89% (iv) (a) 2-aminophenol, EtOH, o/n, rt, then (b) HgO, S, reflux, 2 h, 35–61% (v) 1,1'-carbonyldiimidazole, imidazole, CH3CN, reflux 2-12 h, 50–90% (vi) 2 M aq. NaOH, MeOH, reflux, 30 min, 62%.
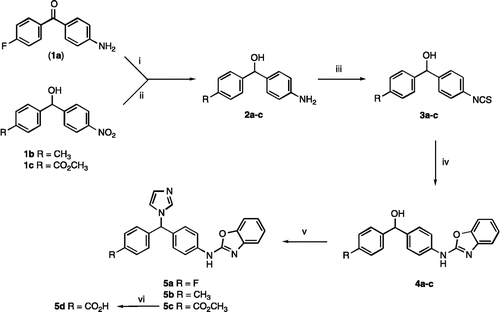
Scheme 2. Reagents and Conditions (i) (a) 2-aminophenol, EtOH, o/n, rt, then (b) HgO, S, reflux, 2 h, 46% (ii) HBr, AcOH, reflux 6 h then o/n rt, 77% (iii) NaBH4, MeOH, 1–12 h, rt, 42–91% (iv) 1,1′-carbonyldiimidazole, imidazole, CH3CN, reflux 2–12 h, 38–74%.
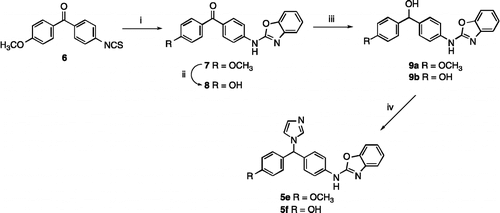
Scheme 3. Reagents and Conditions (i) 1H-1,2,4-triazole, SOCl2, K2CO3, CH3CN, 72 h, rt,11 35%, 12 12% (ii) 1H-tetrazole, SOCl2, K2CO3, CH3CN, 72 h, rt, 32%.
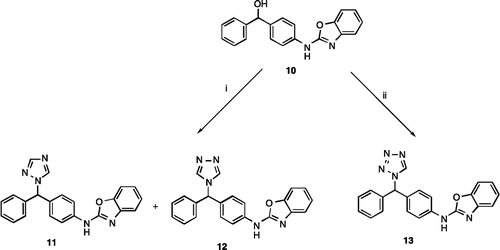
Table II. IC50 data for the novel imidazole N-phenylbenzo[d]oxazolamines (5) .
.
Table III. IC50 data for the novel triazole and tetrazole derivatives compared with imidazoleA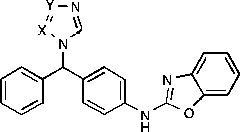
![Figure 1. Structure and key binding interactions of designed N -phenylbenzo[d]oxazolamines.](/cms/asset/b805bbb7-c3af-4a1e-bde0-a699265307b7/ienz_a_322000_f0001_b.gif)
![Figure 2. Disconnective strategy toward substituted N-phenylbenzo[d]oxazolamines.](/cms/asset/a466685b-0539-49f1-900f-5535fa026a41/ienz_a_322000_f0002_b.gif)