Figures & data
Figure 1. General structure of long-chain arylpiperazine (LCAPs) and their representatives of 5-HT1A and 5-HT7 receptor ligands.
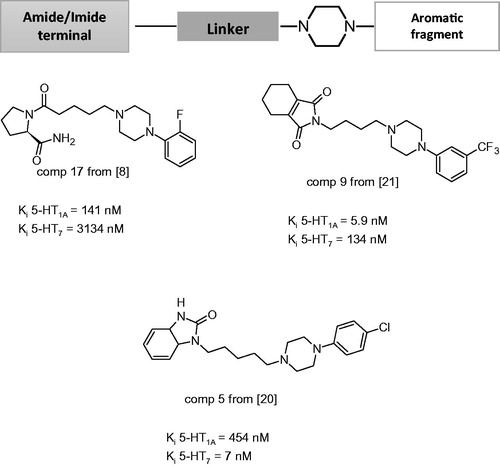
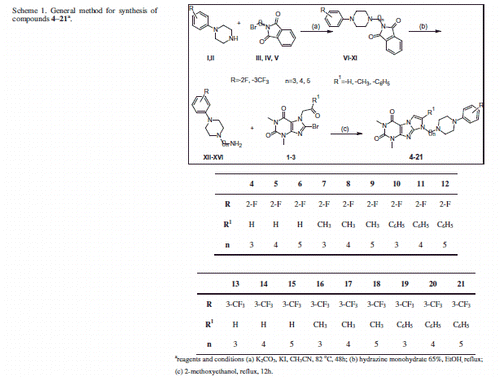
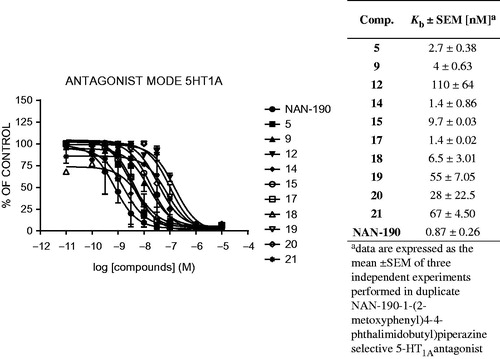
Table 1. The binding data and cLog p of compounds 4–21.
Figure 3. Inhibitor activity (%) against PDE10A for compound 9, theophylline and papaverine in concentration of 10–5 M and 10–5.5 M.
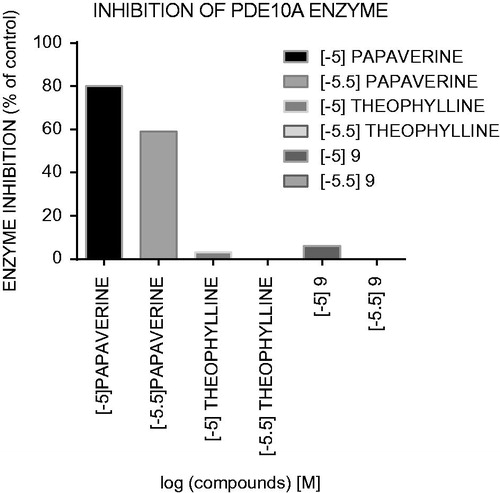
Table 2. Inhibition of PDE (%) of compounds 4–21.
Table 3. Metabolic stability screen of compounds 9 and 20 in human liver microsomes (HLM) and major metabolites characteristics.
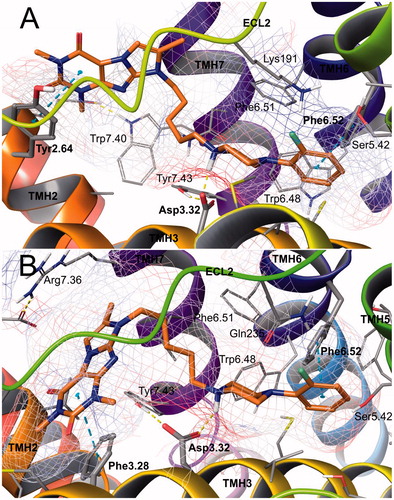
Figure 8. Binding modes of compound 9 in the site of 5-HT1A (A) and 5-HT7 (B) receptors. Amino acid residues engaged in ligand binding (within 4 Å from the ligand atoms) are displayed as sticks, whereas those forming H-bonds (dotted yellow lines) or π–π/CH-π stacking (dotted cyan lines) are represented as thick sticks. Van der Waals molecular surface of the receptor binding site is shown as grid, colored due to electrostatic potential. For the sake of clarity, ECL2 residues were hidden. TMH – transmembrane helix; ECL – extracellular loop.