Figures & data
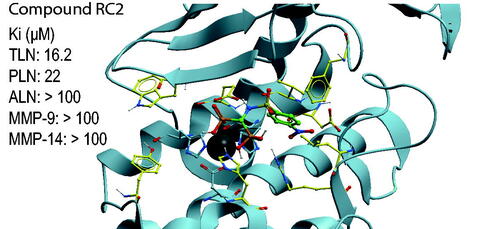
Figure 1. Inhibitory effect of 100 μM of the catechol containing compounds on the activity of the human metalloproteases, MMP-9 and MMP-14, and the bacterial metalloproteases TLN, PLN and ALN. The inhibition experiments were performed by using a fixed concentration of 4 μM of both the MMP-9 and MMP-14 substrate McaPLGL(Dpa)AR-NH2 and the ALN, PLN and TLN substrate McaRPPGFSAFK(Dnp)-OH. The vi/v0 (mean ± sd) were based on 4–6 experiments.
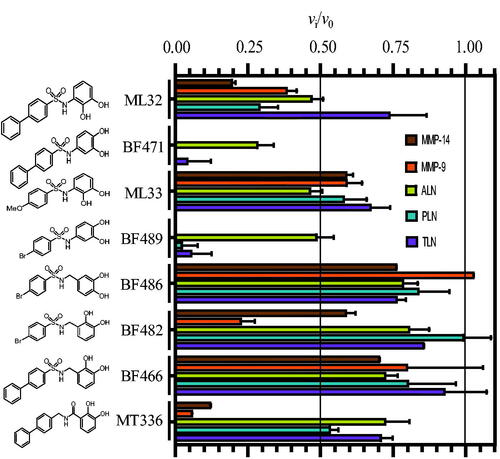
Table 1. Ki values of the catechol containing compounds for TLN, PLN, ALN, MMP-14, and MMP-9(T).
Figure 2. Dose response plot of BF482 for MMP-9(A). The enzyme with and without inhibitor was pre-incubated for 30 min at room temperature and the reaction was started by adding McaPLGL(Dpa)AR-NH2 (4 μM in assay). The reaction was allowed to proceed for 4 h at 37 °C and stopped by the addition of EDTA (10 mM end concentration). The relative fluorescence was determined with the Clariostar plate reader as described in the Experimental Section, where vi and v0 are the reaction rates in the presence and absence of BF482, respectively. Each point on the curve shows the mean ± sd (N = 5 for all points except for two points where N = 4). The regression coefficient r2 is 0.97, with a determined IC50 value of 38.8 ± 0.9 μM and a Ki value of 19.4 ± 0.4 μM.
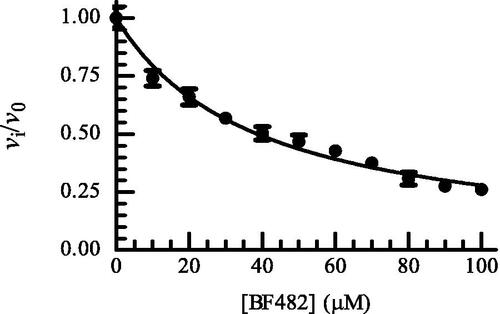
Figure 3. BF471 docked into PLN, TLN, ALN and MMP-9, and ML32 docked into ALN and MMP-9. Zinc is shown in dark grey. The side chain of zinc coordinating amino acids are shown in blue. The side chains of some amino acids of importance for ligand binding are displayed with the following colour coding of atoms: oxygen: red; nitrogen: blue, carbon: yellow, hydrogen: grey. Colour coding of ligand atoms: oxygen: red; sulphur: green; nitrogen: blue; hydrogen; grey; carbon (BF471): pink; carbon (ML32): orange.
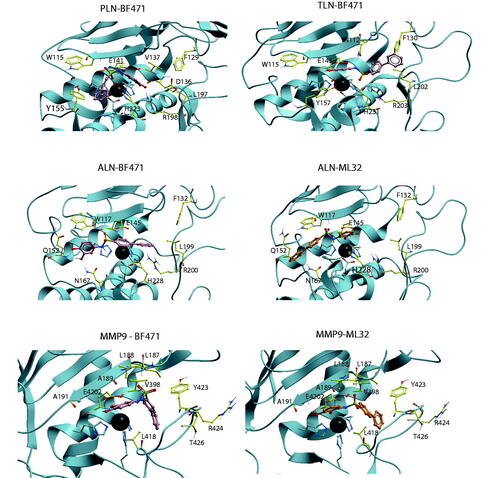
Table 2. Functionally important amino and amino acids in the S1-, S1′- and S2′-subpockets of TLN, PLN, ALN, MMP-9, and MMP-14 contributing to the binding of catechol or bisphosphonate containing compounds tested in the present study.
Figure 4. Inhibitory effect of 100 μM bisphosphonate containing compounds on the activity of the human metalloproteases, MMP-9 and MMP-14, and the bacterial metalloproteases TLN, PLN, and ALN. The inhibition experiments were performed by using a fixed concentration of 4 μM of both the MMP-9 and MMP-14 substrate McaPLGL(Dpa)AR-NH2 and the ALN, PLN and TLN substrate McaRPPGFSAFK(Dnp)-OH. The vi/v0 (mean ± sd) were based on 4–6 experiments.
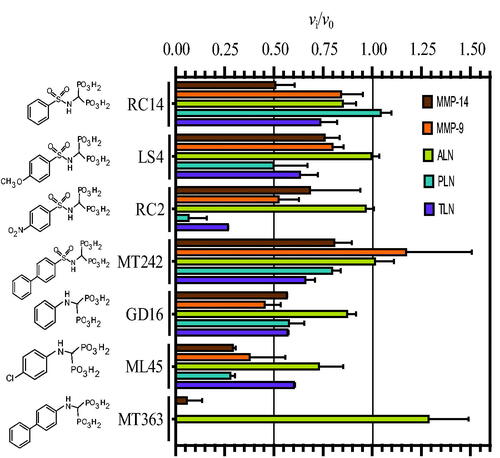
Table 3. The obtained Ki values of the various bisphosphonate containing compounds for TLN, PLN, ALN, MMP-14 and MMP-9.
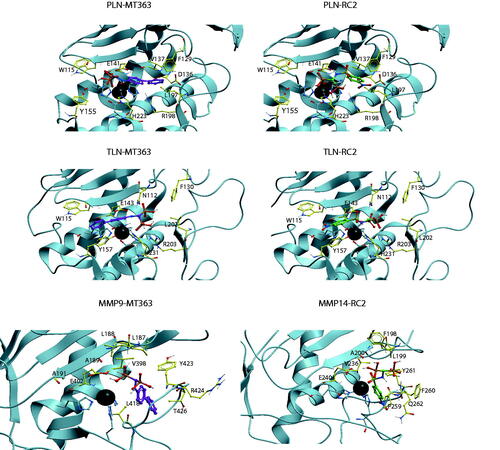
Figure 5. MT363 and RC2 docked into PLN, TLN MMP-9 (MT363) and MMP-14 (RC2). TLN, PLN and MMP-9. Zinc is shown in dark grey. The side chain of zinc coordinating amino acids are shown in blue. The side chains of some amino acids of importance for ligand binding are displayed with the following colour coding of atoms: oxygen: red; nitrogen: blue; carbon: yellow; hydrogen: grey. Colour coding of ligand atoms: oxygen: red; sulphur: green; nitrogen: blue; phosphate: orange; hydrogen: grey; carbon – MT363: purple; carbon – ML32: orange.