Figures & data

Figure 1. (A) Structure of one Kv1.5 α-subunit with six membrane-spanning domains and the intracytoplasmic accessory β-subunits. (B) α and accessory β-subunits co-assemble as tetramers to form the functional channel.
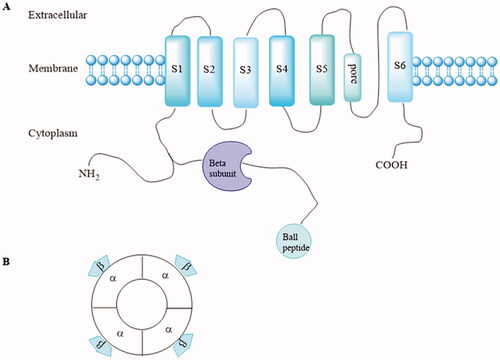
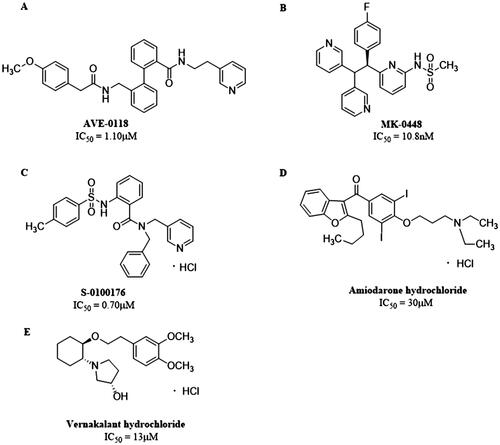
Figure 2. Structures of (A) AVE-0118. (B) MK-0448. (C) S-0100176. (D) Amiodarone hydrochloride. (E) Vernakalant hydrochloride.
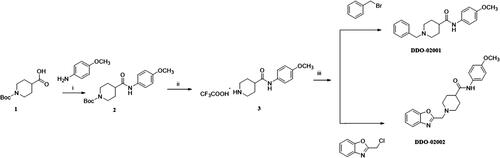
Scheme 1. Synthesis of DDO-02001 and DDO-02002. Reagents and conditions: (i) EDCI, DMAP, THF, r.t.; (ii) CF3COOH, DMF, r.t.; (iii) K2CO3, CH3CN, 80 °C, reflux.
Scheme 2. Synthesis of DDO-02003-DDO-02009. Reagents and conditions: (i) piperidin-4-one-hydrochloride, K2CO3, r.t.; (ii) NaB(OAc)3H, anhydrous. ClCH2CH2Cl, r.t.
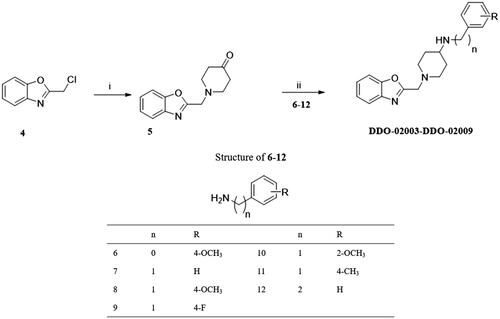
Table 1. Inhibition activities of DDO-02002-DDO-02009 on Kv1.5 channel 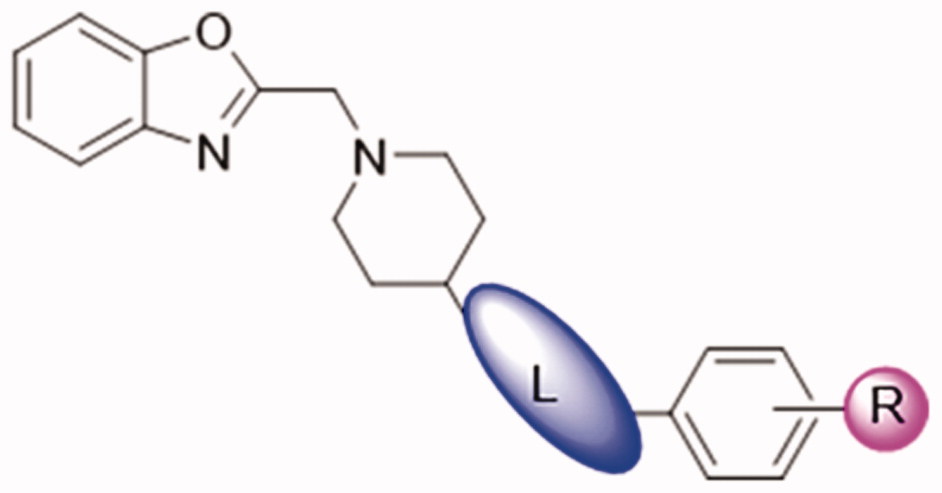
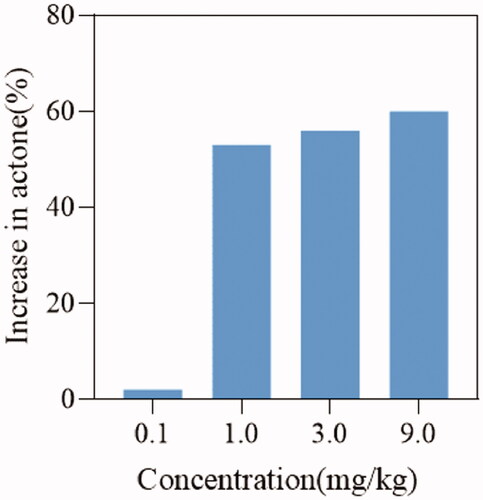
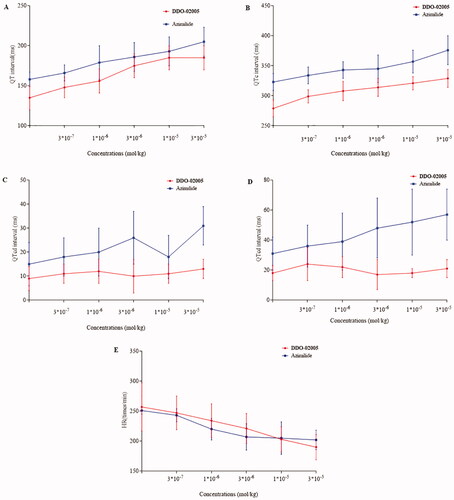
Figure 4. Effect of compound DDO-02005 and dronedarone on (A) atrial fibrillation, (B) atrial ERP and (C) ventricle ERP. Values of *p < 0.05, **p < 0.01 and ***p < 0.001 were considered statistically significant.
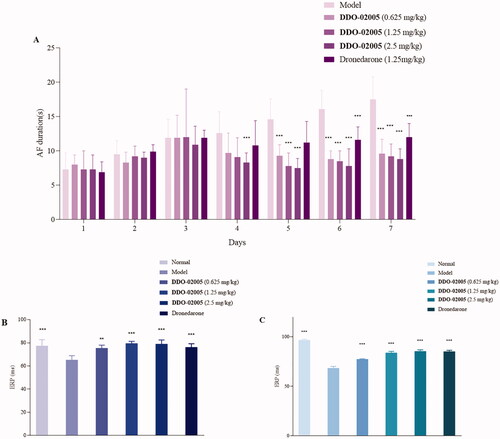
Table 2. Pharmacokinetic parameters regarding lead compound DDO-02005 (mean ± SD, n = 6)