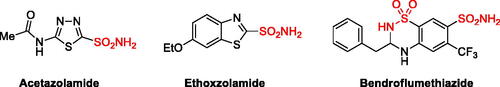
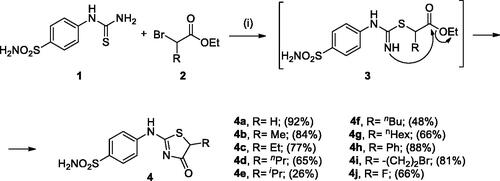
Figures & data
Figure 2. (a) General structure of thiazolone-benzenesulphonamides as selective hCA IX inhibitors developed by Hassan Citation12; (b) General structure of thiazolone-benzenesulphonamides discussed in the paper.
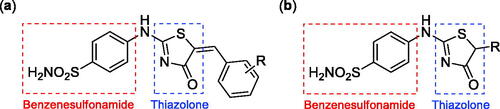
Table 1. Inhibition data of human CA isoforms hCA I, II, and VII and bacterial β-CA isoforms MscCA, from Mammaliicoccus (Staphylococcus) sciuri, and StCA1 and StCA2, from Salmonella enterica (serovar Typhimurium) with compounds 4a–j in comparison with the standard sulphonamide inhibitor AAZ by a stopped flow CO2 hydrase assay.