Figures & data
Figure 1. HPLC-DAD chromatograms of (A) GPE, (B) DPE, and (C) PPE, registered at 280 nm. Pinocembrin RT = 18.89; chrysin RT = 19.43, galangin RT = 21.05, kaempferol RT = 21.57.
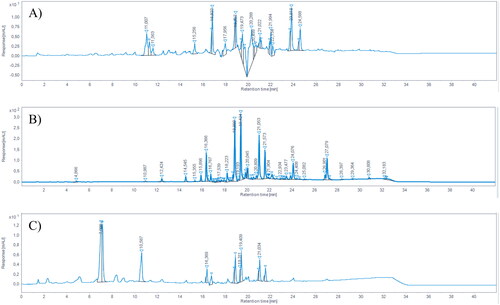
Table 1. Chemical characterisation of the extracts. Values (mg/g dry propolis) are expressed as mean ± standard deviation.
Table 2. Relative gastric stability of reference standards. Values (%) are expressed as mean ± standard deviation.
Figure 2. Relative gastric stability of GPE, DPE, and PPE flavonoids. ***p < 0.001 vs reference standard; °°°p < 0.001 DPE vs GPE and PPE; ##p < 0.01 PPE vs GPE; two-way ANOVA followed by Tukey’s post-hoc.
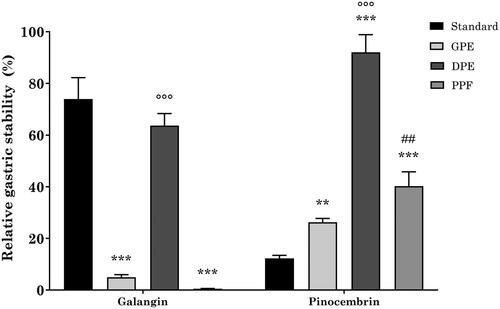
Table 3. Anti-HP activity of DPE and its constituents against the VacA + CagA + 10K clinical isolate and the VacA + CagA- G21 strain. Amoxicillin, clarithromycin, and metronidazole were used as reference drugs. MIC and MBC are expressed as mg/L.
Table 4. Urease inhibition by DPE, galangin, and pinocembrin at the respective MBC and IC50.
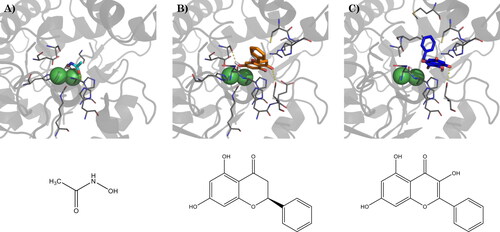
Figure 3. (A) Binding mode of acetohydroxamic acid (cyan) in the active site of HP urease (PDBID: 1e9y), and best docking pose of (B) pinocembrin and (C) galangin. Interacting residues are shown as gray lines. Hydrogen bonds are represented by yellow dashed lines. Bottom: schematic representation of acetohydroxamic acid, pinocembrin, and galangin, respectively.