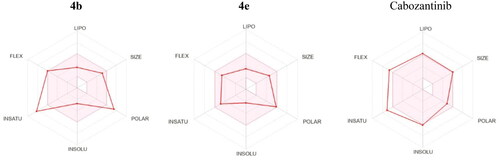
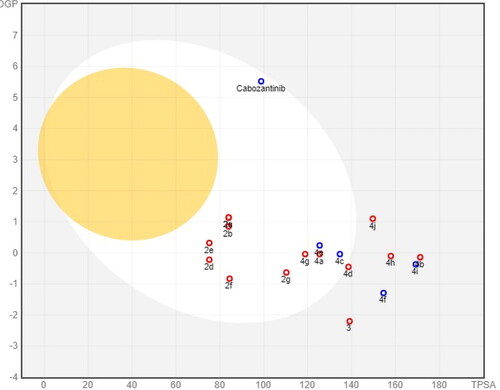
Figures & data
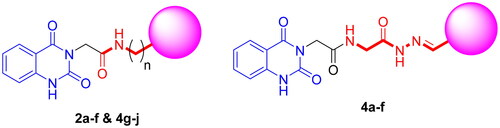
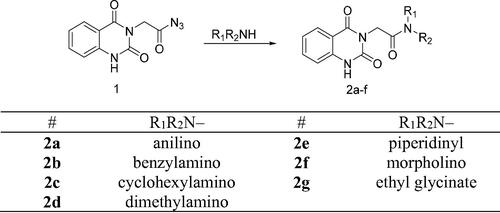
Scheme 2. Synthesis of target compounds 3 and 4a–j; reagents and conditions: (a) N2H4·H2O, EtOH, reflux. (b) Ar-CHO, piperidine, EtOH, reflux. (c) Cyclohexanone, EtOH, reflux. (d) Isatin, AcOH, reflux. (e) Acetylacetone, EtOH, reflux. (f) CS2, pyridine, reflux. (g) PhNCS, EtOH, reflux. (h) NaOH.
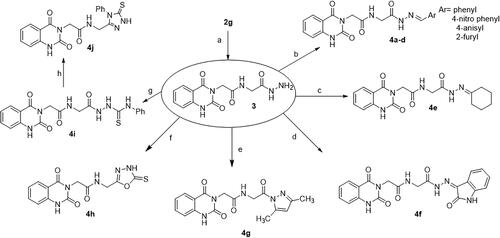
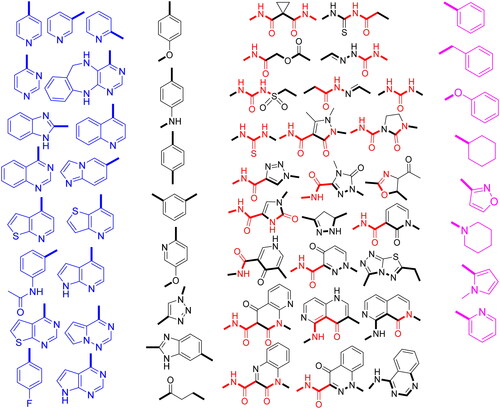
Table 1. In vitro cytotoxicity of compounds 2a–g, 3, 4a–j, and cabozantinib against HCT116 cell line.
Table 2. In vitro cytotoxicity of compounds 4b and 4e against WI38 normal cell line.
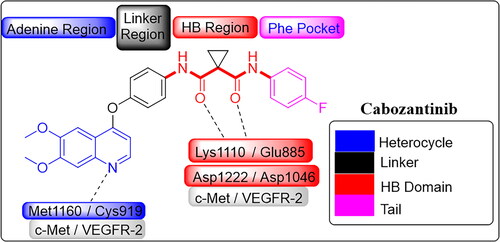
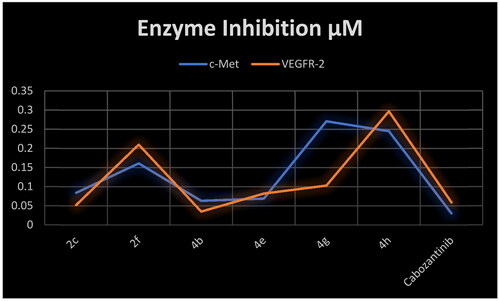
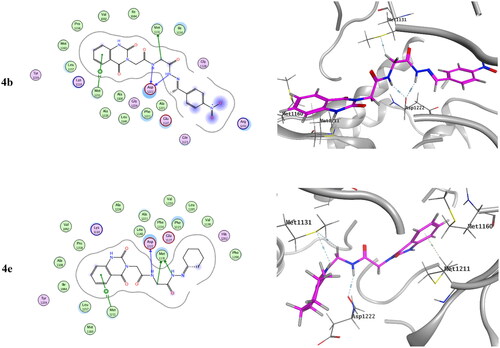
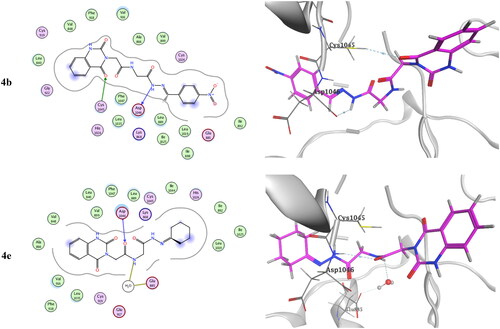
Table 3. Inhibitory activity of selected compounds against c-Met and VEGFR-2.
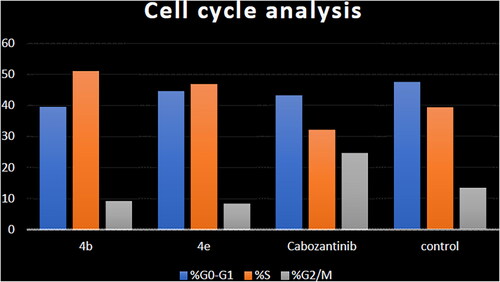
Table 4. Cell cycle analysis in HCT-116 colon cancer cell line treated with compounds 4b and 4e.
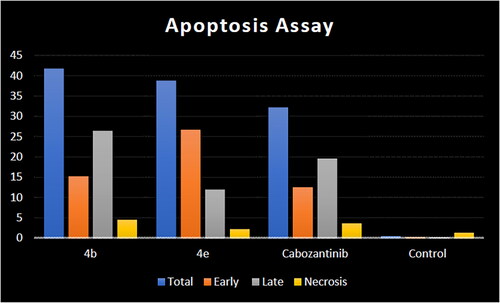
Figure 10. Representative cytograms of apoptotic HCT-116 cells induced by 4b and 4e compared to cabozantinib for 24 h.
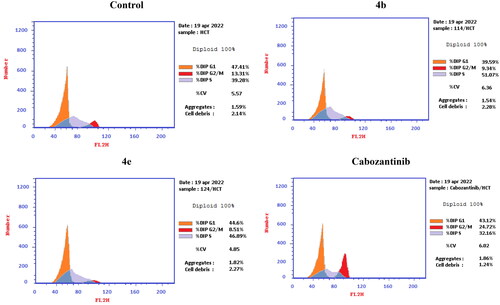
Figure 11. Representative cytograms of apoptotic HCT-116 cells induced by 4b and 4e compared to cabozantinib for 24 h.
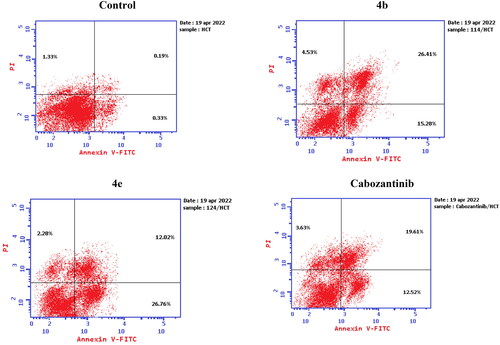
Table 5. Apoptosis induction analysis for compounds 4b, 4e, and cabozantinib.