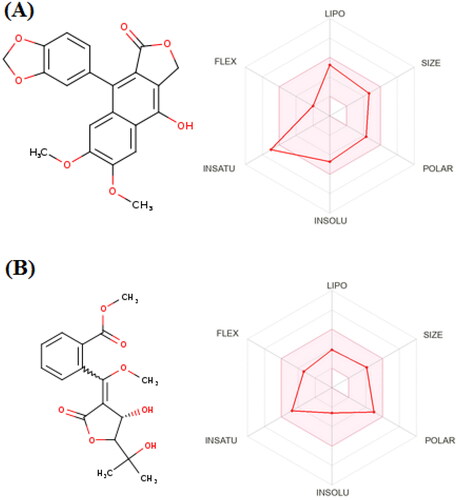
Figures & data
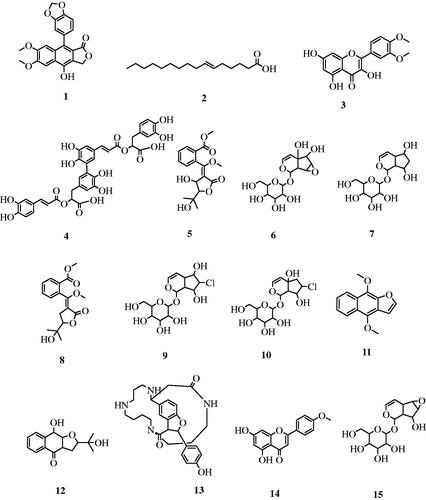
Table 1. LC-HRESIMS-dereplicated phytochemicals in the methanol extract of Thunbergia grandifolia.
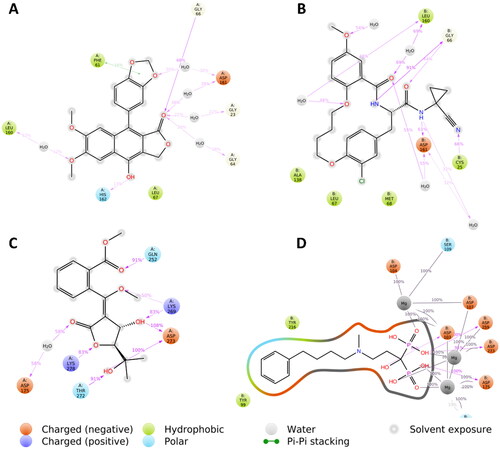
Figure 2. Binding modes of diphyllin (left side) inside the active site of rhodesain target. Binding mode of co-crystalized ligand (right side) inside the active site of rhodesain.
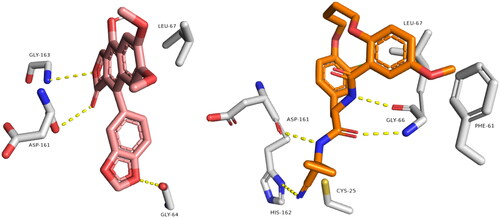
Figure 3. Binding modes of avicennone B (left side) inside the active site of farnesyl diphosphate synthase target. Binding mode of co-crystalized ligand (right side) inside the active site of farnesyl diphosphate synthase.
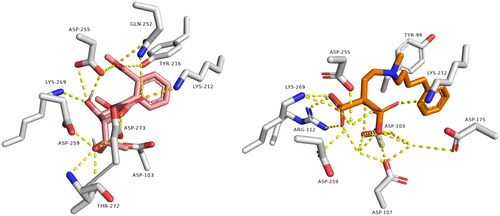
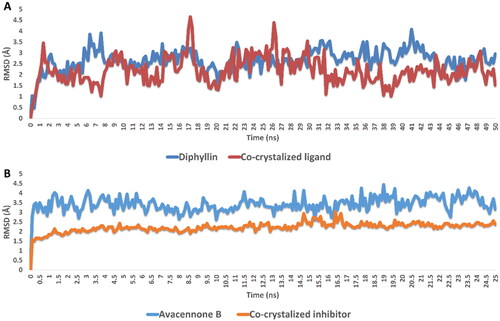
Figure 4. RMSDs of diphyllin and avacennone B inside the active sites of rhodesain and farnesyl diphosphate synthase, respectively, over 50 ns of MDS.