Figures & data
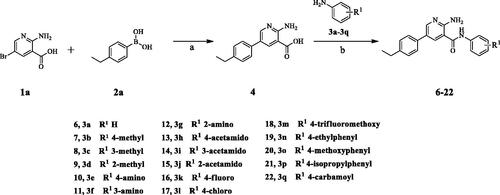
Scheme 1. Synthesis of aryl amide (6–22). Reagents and conditions: (a) 1a (1.0 eq.), 2a (1.0 eq.), K3PO4 (1.5 eq.), Pd(PPh3)4 (0.05 eq.), 80 °C, 1,4-dioxane/H2O (3:1), Ar2 atmosphere, 12 h; (b) 5 (1.0 eq.), aniline (1.1 eq.), DIPEA (4.0 eq.), HATU (1.2 eq.), room temperature, DCM, 18 h.
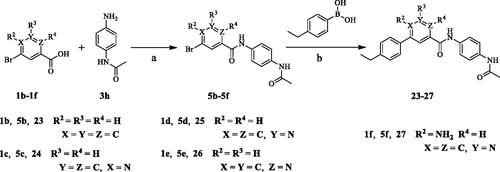
Scheme 2. Synthesis of aryl amide (23–37). Reagents and conditions: (a) Acid (1.0 eq.), 3h (1.0 eq.), HATU (1.2 eq.), DIPEA (4.0 eq.), RT, DCM, 18 h; (b) Bromide (1.0 eq.), 2a (1.0 eq.), K3PO4 (1.5 equiv), Pd(PPh3)4 (0.05 eq.), 80 °C, Ar2 atmosphere, 1,4-dioxane/H2O (3:1), 12 h.
Scheme 3. Synthesis of aryl amide (28–52). Reagents and conditions: (a) 1a (1.0 eq.), 3H (1.0 eq.), HATU (1.2 eq.), DIPEA (4.0 eq.), RT, DCM, 18 h; (b) 4a (1.0 eq.), boric acid (1.0 eq.), K3PO4 (1.5 equiv), Pd(PPh3)4 (0.05 eq.), 1,4-dioxane/H2O, Ar2 atmosphere, 80 °C, 12 h.
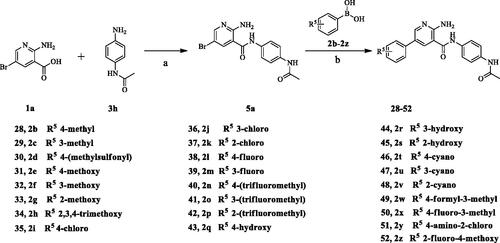
Table 1. Structure-activity relationship study at position 3 of the pyridine core.
Table 2. Structure-activity relationship study of the core.
Table 3. Structure-activity relationship study at position 5 of the pyridine core.
Figure 2. Compound 51 suppressed the activation of NF-κB. (A–C) Compound 51 inhibited the phosphorylation of P65 and IκBα. RAW264.7 cells were treated with compound 51 for different concentrations and then stimulated by LPS. The cell lysates were analysed using WB. ###p < 0.001 compared with control group. **p < 0.01, ***p < 0.001 compared to LPS group. (D–F) Compound 51 inhibited TNFα-induced nuclear translocation of p65 and p50. HEK293T were treated with compound 51 for different concentrations and then stimulated by TNF-α. The nuclear protein was analysed using WB. ###p < 0.001 compared with control group. **p < 0.01, ***p < 0.001 compared to TNF-α group.
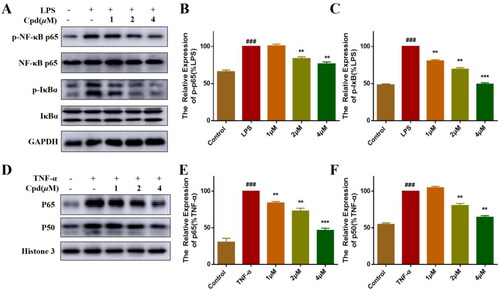
Figure 3. Compound 51 inhibited the activation of MAPKs signalling. (A–D) Compound 51 inhibited the phosphorylation of MAPKs. RAW264.7 cells were treated with compound 51 for different concentrations and then stimulated by LPS. The cell lysates were analysed using WB. ###p < 0.001 compared with control group. *p < 0.1, **p < 0.01, ***p < 0.001 compared to LPS group.
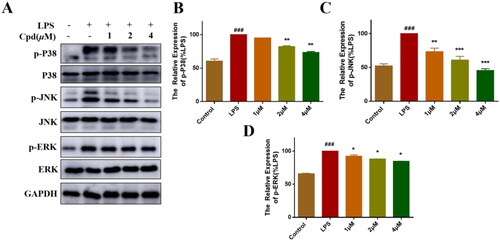
Figure 4. Compound 51 inhibited LPS-induced inflammatory response. (A–C) The expression of iNOS and COX-2. RAW264.7 cells were treated with compound 51 for 1 h, then stimulated by LPS for further 24 h. The samples were analysed by WB. (D). The level of IL-6. (E) The level of TNF-α. (F) The level of ROS. ###p < 0.001 compared with control group. *p < 0.1, **p < 0.01, ***p < 0.001 compared to LPS group.
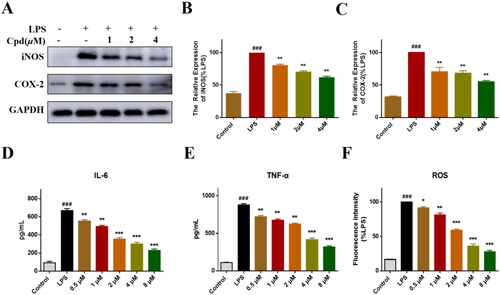
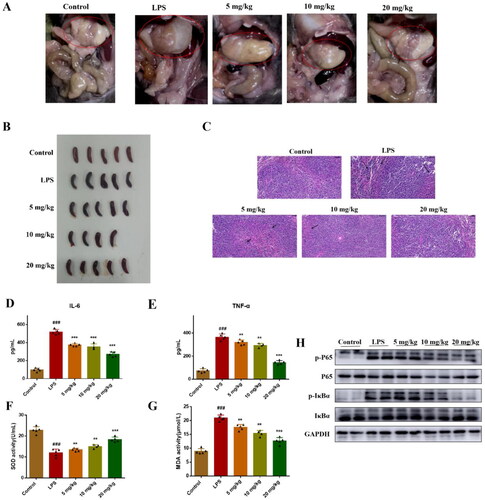
Figure 5. Compound 51 suppressed LPS-induced inflammatory response in vivo. (A-B) The picture of mice organs. (C) HE staining of spleen tissues. Scan bar:50 μM. (D-E) The levels of IL-6 and TNF-α in blood. The samples were analysed by ELISA kits. (F–G) The SOD and MDA activity in blood. ###p < 0.001 compared with control group. *p < 0.1, **p < 0.01, ***p < 0.001 compared to LPS group. (H) The expression of NF-κB in splenic organ. The samples were analysed by WB.