Figures & data
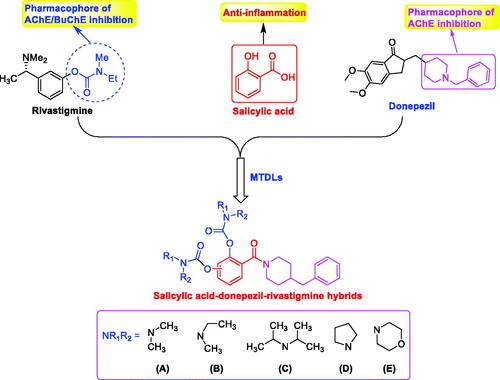
Scheme 1. Synthesis of 5a–5h. Conditions: (i) EDCI, HOBT, THF, and room temperature. (ii) N,N-disubstituted carbamoyl chlorides (4a–4e), CH3CN, 65 °C.
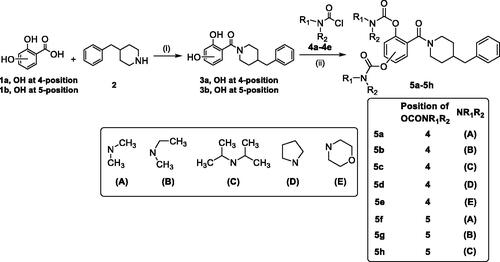
Table 1. The inhibitory capacity of AChE/BuChE by 5a–5h, rivastigmine and donepezil.
Figure 2. (A) BuChE recovery capability after preincubation of compound 5a was diluted to 0.1 × IC50. (B) The recovery capability of BuChE inhibition by rivastigmine, donepezil, and 5a was diluted to 0.1 × IC50 by monitoring with time for 240 min. The results were indicated as the mean ± SEM.
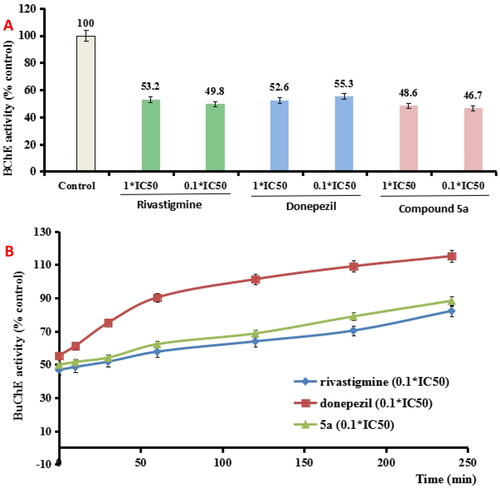
Figure 3. (A) 5a (green stick) interacted with the binding residues of huBuChE (PDB code: 4tpk). (B) 2D interaction of 5a (green stick) with huBuChE.
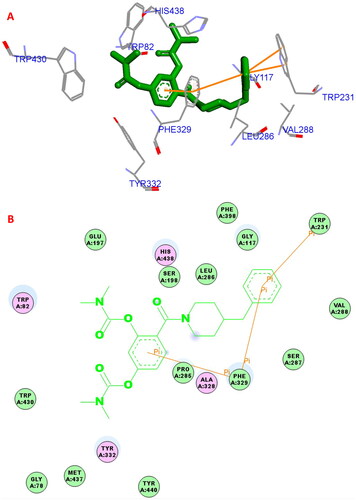
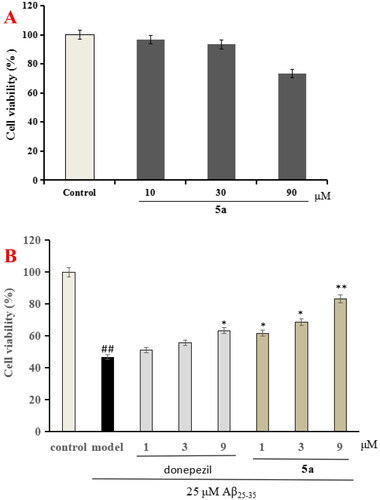
Figure 4. The cell viability was determined using CCK-8 assay. The results were indicated as the mean ± SD.
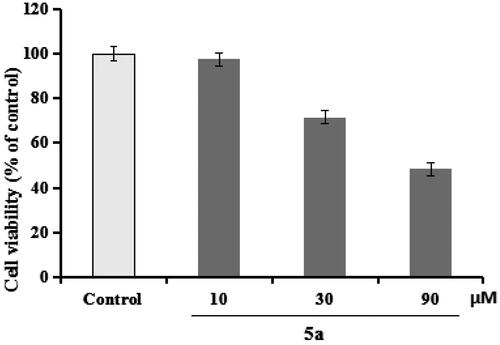
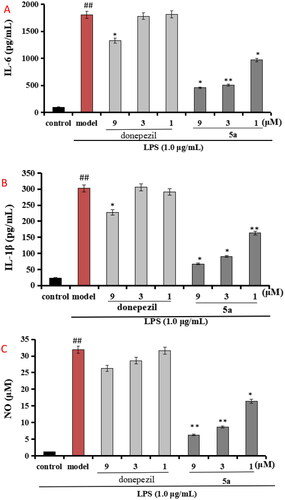
Figure 5. (A) Effects of compounds 5a and donepezil on the production of IL-6; (B) effects of compound 5a and donepezil on the production of IL-1β; (C) effects of compound 5a and donepezil on NO release. The results were expressed as the mean ± SD. *p < 0.05, **p < 0.01 vs. model group; ##p < 0.01 vs. control.