Figures & data
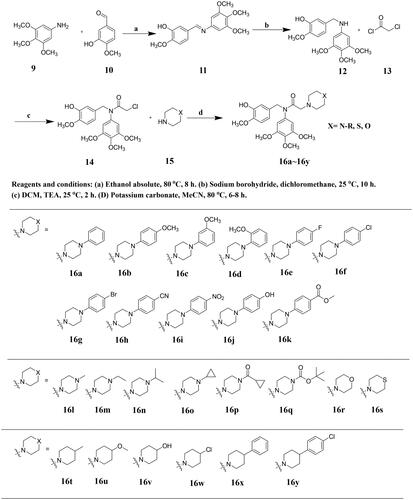
Table 1. Antiproliferative activities of compounds 16a–16k and colchicine.
Table 2. Antiproliferative activities of compounds 16l–16s and colchicine.
Table 3. Antiproliferative activities of compounds 16t–16y and colchicine.
Table 4. Antiproliferative activities of compound MY-1121 against six additional cancer cell lines.
Figure 4. Effects of compound MY-1121 on tubulin polymerisation under cell free condition. The vertical coordinate indicates the degree of tubulin polymerisation. The experiments were repeated thrice.
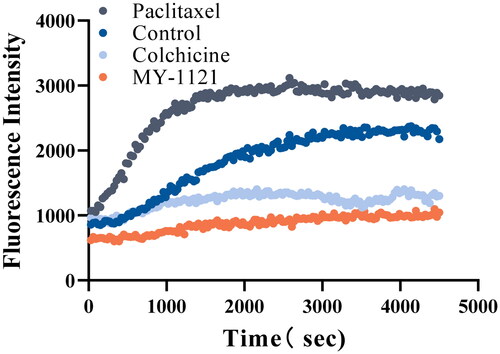
Figure 5. Compound MY-1121 bind to β-tubulin directly on colchicine binding site. (A&B). EBI competition assay, the affinity of the compound with colchicine binding site was negatively correlated with the level of the tubulin adduct band; (C&D). Alkaline protease assay. Cells were treated with Alkaline Proteinase and different concentration of compound MY-1121. The band signal of β-tubulin was negatively correlated with the ability of Alkaline Protease to hydrolyze β-tubulin. The binding of the compound to β-tubulin was able to inhibit the hydrolysis of β-tubulin by Alkaline Protease.
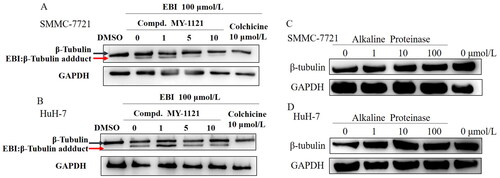
Figure 6. Effects of compound MY-1121 on microtubule network in liver cancer cells SMMC-7721 and HuH-7. Cells were treated for 48 h. β-tubulin was stained green and cell nuclei were stained blue.
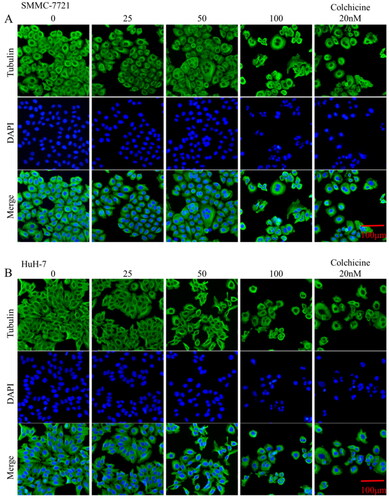
Figure 7. Molecular docking results of compound MY-1121 with tubulin (PDB: 1AS0). The hydrogen bond, ionic interactions, and hydrophobic interactions are depicted as yellow, magentas, and green dashed lines, respectively.
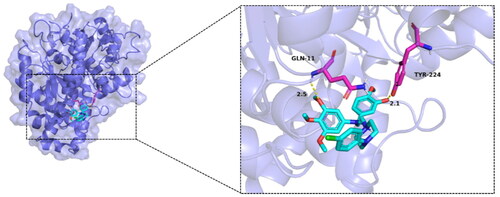
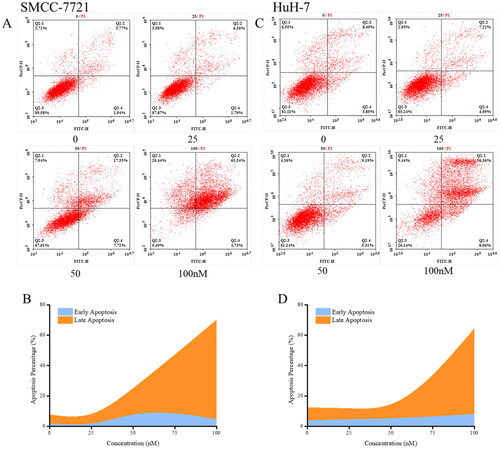
Figure 8. Effects of compound MY-1121 on cell cycle distribution. Liver cancer cells SMMC-7721 (A, B) and HuH-7 (C, D) were treated for 48 h. The cells were stained with propidium iodide and analysed via flow cytometry to measure the cell cycle profile.
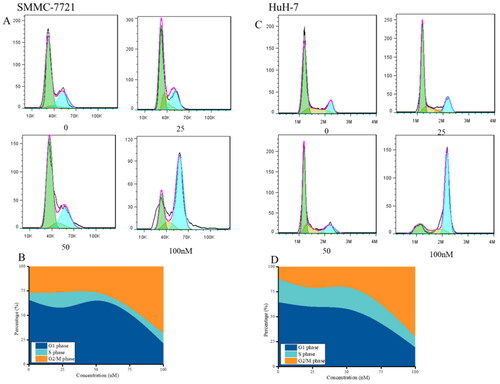
Figure 9. Effects of compound MY-1121 on colony formatting ability. Liver cancer cells SMMC-7721 (A) and HuH-7 (B) were treated for seven days.
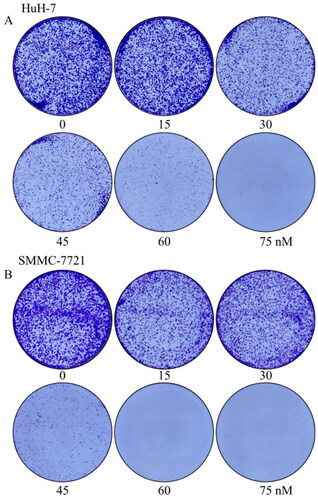
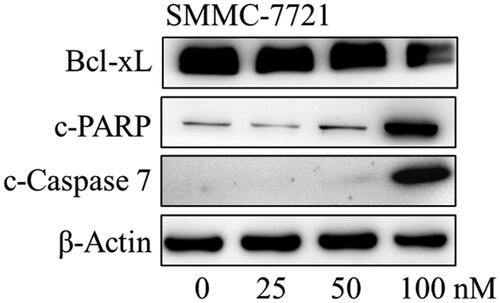
Figure 10. The effects of compound MY-1121 on cell cycle related proteins in SMMC-7721 cell were conducted via Western blotting assay. Cells were treated with indicated concentrations of compound MY-1121 for 48 h.
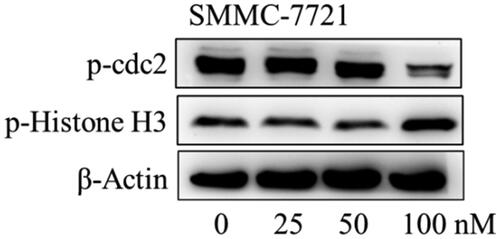
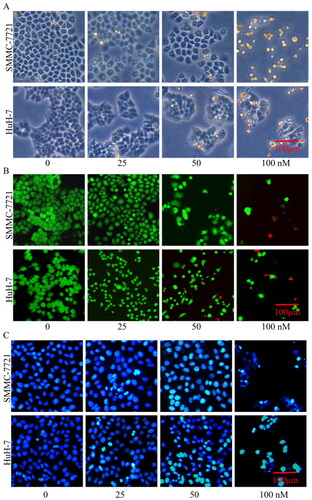
Figure 11. Effects of compound MY-1121 on morphology changes of liver cancer cells. Liver cancer cells were treated for 48 h. (A) Cell morphology in the bright field; (B) number of live cells (green) to dead cells (red); and (C) cell nuclei changes.