Figures & data
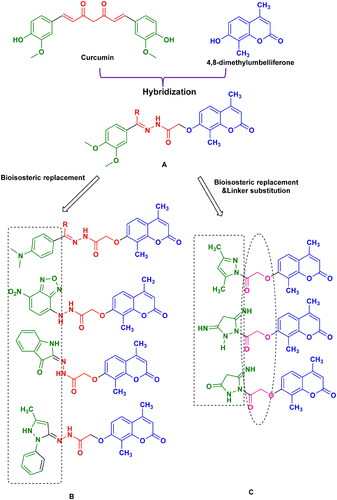
Figure 1. Design of coumarin-based analogs combined with curcumin derivatives and other different nitrogenous heterocyclic cores as anti-inflammatory candidates.
Scheme 1. Synthetic pathways for preparation of starting materials 3 and 4a–c. Reagents: a) ethyl chloroacetate, anhydrous K2CO3, acetone, reflux 24 h; b) hydrazine hydrate, absolute ethanol, reflux 2 h; c) 30% NaOH, ethanol, stirring r.t., 2 h.
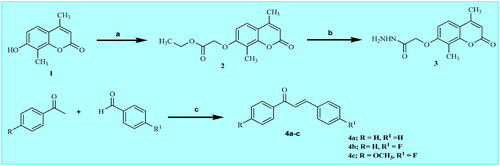
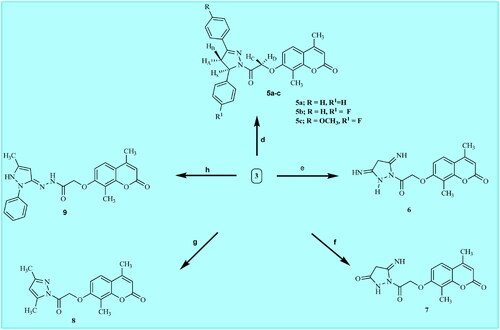
Scheme 2. Synthetic pathways for preparation of target coumarin derivatives 5a-c and 6–9. Reagents: d) chalcone 4a-c, NaOH, absolute ethanol, reflux 72 h; e) malononitrle, absolute, ethanol, reflux 8 h; f) ethylcyanoacetate, absolute ethanol, reflux 9 h; g) acetylacetone, absolute ethanol, reflux 15 h; h) 3-methyl-1-phenyl-2-pyrazoline-5-one, dioxane, reflux 12 h.
Scheme 3. Synthetic pathways for preparation of target coumarin derivatives 10–12, 13a,b and 14a,b. Reagents: i) 4-chloro-7-nitrobenzo[c][1,2,5]oxadiazole, absolute ethanol, reflux 8 h; j) tosyl chloride, dioxane, reflux 8 h; k) isatin, absolute ethanol, glacial acetic acid reflux 8 h. l) appropriate aryl aldehyde, absolute ethanol, glacial acetic acid, reflux 9–16 h; m) appropriate aryl ketone, absolute ethanol, glacial acetic acid reflux 10–16 h.
![Scheme 3. Synthetic pathways for preparation of target coumarin derivatives 10–12, 13a,b and 14a,b. Reagents: i) 4-chloro-7-nitrobenzo[c][1,2,5]oxadiazole, absolute ethanol, reflux 8 h; j) tosyl chloride, dioxane, reflux 8 h; k) isatin, absolute ethanol, glacial acetic acid reflux 8 h. l) appropriate aryl aldehyde, absolute ethanol, glacial acetic acid, reflux 9–16 h; m) appropriate aryl ketone, absolute ethanol, glacial acetic acid reflux 10–16 h.](/cms/asset/101c75c9-b3e7-4203-8dff-89429ce4653b/ienz_a_2243551_sch0003_c.jpg)
Table 1. The calculated EC50 and IC50 of the tested compounds on normal and LPS-treated Macrophages.
Figure 2. Represents the impact of coumarin molecule bearing 3,4-dimethoxybenzylidene hydrazinyl 14b compared to negative control and macrophage cells treated with Dexa on the levels of pro-inflammatory cytokines. (A) Effect on IL-6 level. (B) Effect on IL-1β level. (C) Effect on TNF-α level. (D) Effect on NF-kβ level.
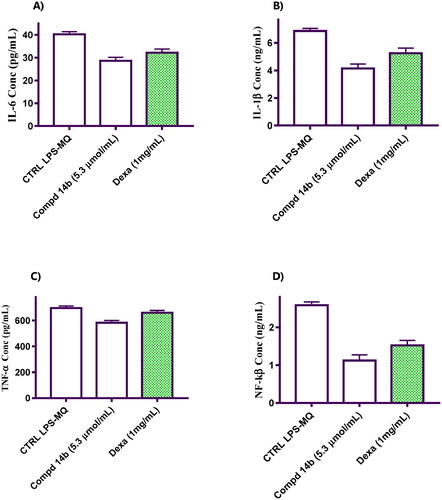
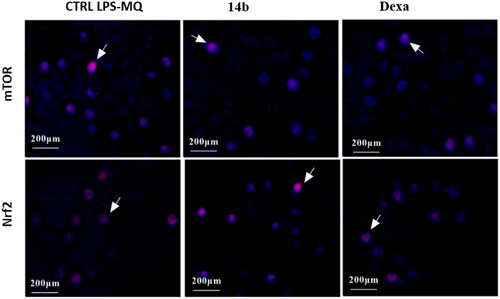
Figure 4. Represents the calculation of H-Score for m-TOR (A) and Nrf-2 (B) protein expression in the tested groups induced by coumarin molecule bearing 3,4-dimethoxybenzylidene hydrazinyl 14b compared to negative control and macrophage cells treated with Dexa.
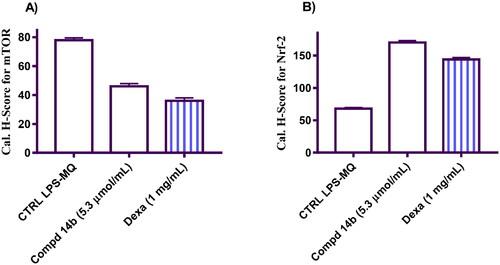
Figure 5. Represents the impact of coumarin molecule bearing 3,4-dimethoxybenzylidene hydrazinyl 14b compared to negative control and macrophage cells treated with Dexa on the gene expression levels of HO-1 and Akt in the tested groups.
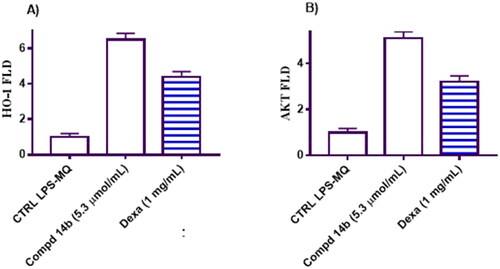
Figure 6. The main proposed action of coumarin derivative 14b to promote anti-inflammatory activity.
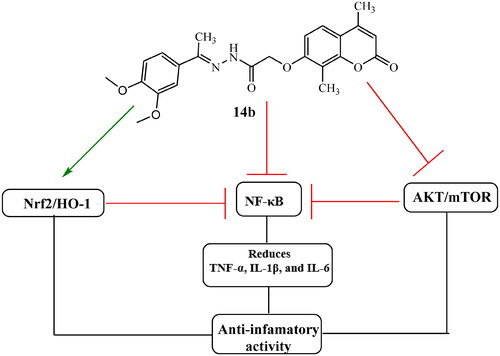
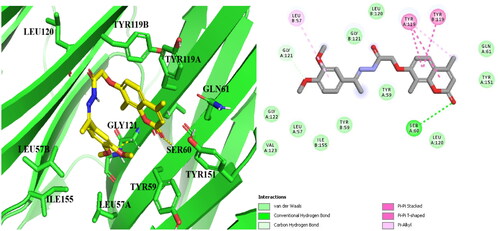
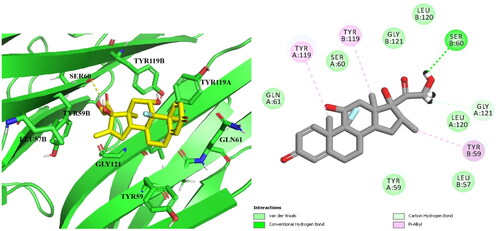
Table 2. Binding score, H-bonding, and Hydrophobic interactions of the target compound 14b, Dexa and co-lig. 307 in the active site of TNF-α.