Figures & data
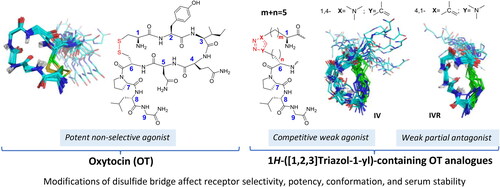
Figure 1. All permutations replacing the disulphide bridge in OT by the side chain-to-side chain 1H-[1,2,3]triazol-1-yl-bridged modification. (A) Partial schematic structure of the 1,4- and 4,1-(1H-[1,2,3]triazol-1-yl) containing permutations included in this study, where m + n = 2–5 and m = 1–4 and n = 1–4 and (B) OT schematic structure.
![Figure 1. All permutations replacing the disulphide bridge in OT by the side chain-to-side chain 1H-[1,2,3]triazol-1-yl-bridged modification. (A) Partial schematic structure of the 1,4- and 4,1-(1H-[1,2,3]triazol-1-yl) containing permutations included in this study, where m + n = 2–5 and m = 1–4 and n = 1–4 and (B) OT schematic structure.](/cms/asset/02ec7ee4-ce8d-4b5e-b350-8c92a738e705/ienz_a_2254019_f0001_c.jpg)
Scheme 1. Sequences of the linear precursors H-Xaa1-Tyr2-Ile3-Gln4-Asn5-Yaa6-Pro7-Leu8-Gly9-NH2 (I’–VII’) and of the 1,4-(1H-[1,2,3]triazol-1-yl) containing cyclopeptides (I–VII), {[H-Ala(&1)-Tyr2-Ile3-Gln4-Asn5-Ala(&2)-Pro7-Leu8-Gly9-NH2]} {[1-[&1(CH2)m]-1H-1,2,3-triazol-4-yl]-(CH2)n&1]} Citation53 with m + n = 5, 3 or 2 and m and n = 1–4, 1–2, or 1. All amino acids are of the L configuration.
![Scheme 1. Sequences of the linear precursors H-Xaa1-Tyr2-Ile3-Gln4-Asn5-Yaa6-Pro7-Leu8-Gly9-NH2 (I’–VII’) and of the 1,4-(1H-[1,2,3]triazol-1-yl) containing cyclopeptides (I–VII), {[H-Ala(&1)-Tyr2-Ile3-Gln4-Asn5-Ala(&2)-Pro7-Leu8-Gly9-NH2]} {[1-[&1(CH2)m]-1H-1,2,3-triazol-4-yl]-(CH2)n&1]} Citation53 with m + n = 5, 3 or 2 and m and n = 1–4, 1–2, or 1. All amino acids are of the L configuration.](/cms/asset/caa55c46-d7af-43f2-90f5-945966121a3f/ienz_a_2254019_sch0001_c.jpg)
Scheme 2. Sequences of the linear precursors H-Yaa1-Tyr2-Ile3-Gln4-Asn5-Xaa6-Pro7-Leu8-Gly9-NH2 (IR’–VIIR’) and of the 4,1-(1H-[1,2,3]triazolyl containing cyclopeptides (IR–VIIR), {[H-Ala(&1)-Tyr2-Ile3-Gln4-Asn5-Ala(&2)-Pro7-Leu8-Gly9-NH2]} {[1-[&1(CH2)m]-1H-1,2,3-triazol-4-yl]-(CH2)n&1]} Citation53 with m + n = 5, 3, or 2 and m and n = 1–4, 1–2, or 1. All amino acids are of the L configuration.
![Scheme 2. Sequences of the linear precursors H-Yaa1-Tyr2-Ile3-Gln4-Asn5-Xaa6-Pro7-Leu8-Gly9-NH2 (IR’–VIIR’) and of the 4,1-(1H-[1,2,3]triazolyl containing cyclopeptides (IR–VIIR), {[H-Ala(&1)-Tyr2-Ile3-Gln4-Asn5-Ala(&2)-Pro7-Leu8-Gly9-NH2]} {[1-[&1(CH2)m]-1H-1,2,3-triazol-4-yl]-(CH2)n&1]} Citation53 with m + n = 5, 3, or 2 and m and n = 1–4, 1–2, or 1. All amino acids are of the L configuration.](/cms/asset/6fc885a1-5cc7-4962-83e8-5d8753395f84/ienz_a_2254019_sch0002_c.jpg)
Scheme 3. General procedure CuAAC intramolecular macrocyclisation of either 1,4- or 4,1-disubstituted-(1H-[1,2,3]triazol-1-yl) containing OT analogues (A and B respectively) carried out in solution (I–VI and IR–VIR) (a)Citation47,Citation56 (R = NH2) or by on-resin microwave-assisted strategy (VII–VIIR) (b)Citation52 (X= tBu; Y = Trt; R = Tentagel® S RAM resin).
![Scheme 3. General procedure CuAAC intramolecular macrocyclisation of either 1,4- or 4,1-disubstituted-(1H-[1,2,3]triazol-1-yl) containing OT analogues (A and B respectively) carried out in solution (I–VI and IR–VIR) (a)Citation47,Citation56 (R = NH2) or by on-resin microwave-assisted strategy (VII–VIIR) (b)Citation52 (X= tBu; Y = Trt; R = Tentagel® S RAM resin).](/cms/asset/b8bd4d6b-327d-4dd3-8fe3-4ec3badef799/ienz_a_2254019_sch0003_c.jpg)
Table 1. Analytical characterisation data for peptide I–VII and IR–VIIR.
Figure 2. Pharmacology of oxytocin analogues. (A) One-point radioligand displacement experiments. Percentage specific displacement of [3H]-OT from the OTR (n = 2) by compounds I–VII and IR–VIIR (10 μM) normalised to control (10 μM OT). Specific displacement is displayed as a heatmap in greyscale ranging from white (0% specific displacement) to black (100% specific displacement); radioligand bound, i.e. [3H]-OT at 0% displacement corresponds to an average of 3775 fmoles/mg protein, respectively. (B) Concentration-dependent displacement of [3H]-OT from the OTR by compounds I (n = 2), IV (n = 2), IVR (n = 3), V (n = 4), VR (n = 2), VII (n = 4) and VR (n = 2). Eleven semi-logarithmic spaced concentrations were measured in the range from 1 nM to 100 μM. Specific binding was calculated by subtracting the non-specific binding (determined with 10 μM OT) from the total binding. The data were normalised to 100% specific binding of [3H]-OT, which refers to an average of 1000–1500 fmoles/mg protein for oxytocin receptor and fitted by nonlinear regression (slope = 1). (C) Ligand induced activation of the Gq-pathway. Concentration-dependent formation of inositol-1-phosphate (IP-1) by compound IV (n = 3) and IVR (n = 3). The ligand induced formation of IP-1 was measured in HEK293 cells (104 cells/well) stable expressing the oxytocin receptor after stimulation for 1h at 37 °C with ligands. Data are mean ± SD of three independent experiments with three technical replicates. (D) Accumulation of IP-1 by stimulation of OTR with OT (30 pM–1 µM) in the absence or presence of 100 nM, 300 nM, 1 µM or 3 µM of IVR. Data are normalised to percentage of maximal receptor activation, measured at the highest OT concentration, and are shown as mean ± SEM (n = 4). (E) Schild regression analysis of IVR at the OTR: A = EC50 of OT alone; A′ = EC50 of OT in presence of IVR; B = logarithm of IVR concentration; Schild slope = 1.0 ± 0.2 (SEM), R2 = 0.96.
![Figure 2. Pharmacology of oxytocin analogues. (A) One-point radioligand displacement experiments. Percentage specific displacement of [3H]-OT from the OTR (n = 2) by compounds I–VII and IR–VIIR (10 μM) normalised to control (10 μM OT). Specific displacement is displayed as a heatmap in greyscale ranging from white (0% specific displacement) to black (100% specific displacement); radioligand bound, i.e. [3H]-OT at 0% displacement corresponds to an average of 3775 fmoles/mg protein, respectively. (B) Concentration-dependent displacement of [3H]-OT from the OTR by compounds I (n = 2), IV (n = 2), IVR (n = 3), V (n = 4), VR (n = 2), VII (n = 4) and VR (n = 2). Eleven semi-logarithmic spaced concentrations were measured in the range from 1 nM to 100 μM. Specific binding was calculated by subtracting the non-specific binding (determined with 10 μM OT) from the total binding. The data were normalised to 100% specific binding of [3H]-OT, which refers to an average of 1000–1500 fmoles/mg protein for oxytocin receptor and fitted by nonlinear regression (slope = 1). (C) Ligand induced activation of the Gq-pathway. Concentration-dependent formation of inositol-1-phosphate (IP-1) by compound IV (n = 3) and IVR (n = 3). The ligand induced formation of IP-1 was measured in HEK293 cells (104 cells/well) stable expressing the oxytocin receptor after stimulation for 1h at 37 °C with ligands. Data are mean ± SD of three independent experiments with three technical replicates. (D) Accumulation of IP-1 by stimulation of OTR with OT (30 pM–1 µM) in the absence or presence of 100 nM, 300 nM, 1 µM or 3 µM of IVR. Data are normalised to percentage of maximal receptor activation, measured at the highest OT concentration, and are shown as mean ± SEM (n = 4). (E) Schild regression analysis of IVR at the OTR: A = EC50 of OT alone; A′ = EC50 of OT in presence of IVR; B = logarithm of IVR concentration; Schild slope = 1.0 ± 0.2 (SEM), R2 = 0.96.](/cms/asset/5f9b4317-8a00-47b8-b998-8dcd51417de2/ienz_a_2254019_f0002_b.jpg)
Table 2. Pharmacological properties of clicked OT analogues at OTR.
Table 3. Antagonist properties of oxytocin analogues IVR at OTR.
Figure 3. Serum stability. Stability of OT native, IV and IVR analogues in woman serum at 40th week of pregnancy (n = 2).
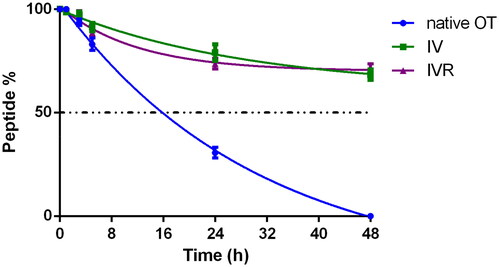
Figure 4. Superimposition of the 20 lowest energy structures of OT and analogues in water. Backbone atoms are shown with the following colours: carbon in cyan, nitrogen in blue, oxygen in red, amide hydrogen in white. The side-chain atoms of the bridging residues (1,6) are also displayed (carbon in green, sulphur in yellow). When present, hydrogen bonds are highlighted in black. As in OT, analogues IR, II and IIR, IVR, V and VR, VI and VIR, and VII and VIIR adopt the type I β-turn conformation centred on Ile(3)-Gln(4) (the H-bond between Tyr(2) CO and Asn(5) HN groups is highlighted in black). Peptide I exhibits a H-bond between residue 6 amide proton and one nitrogen of the triazole ring. Peptides III, IIIR and VIR present an inverse γ-turn on Asn(5). Peptide IV has a flexible skeleton.
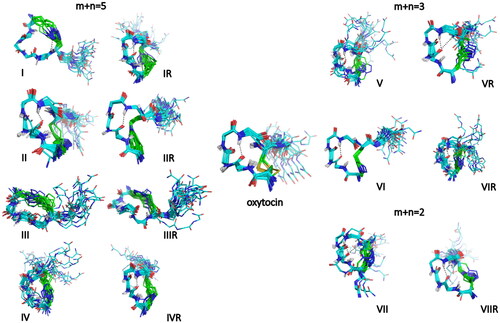
Figure 5. Superimposition of peptides IIR (yellow), IV (orange), IVR (pink) onto OT (cyan) in the OTR receptor (PDB 7QVM). Residues of OTR in close contact with OT ligand (distance < 5 Å) are coloured in green. Only the side chains of W188, M315 and L316 are shown. Analogues II, IV, IVR were modelled by constraining dihedral angles to adopt the bioactive conformation of OT. The 1H-[1,2,3]triazol-1-yl ring in peptides II, IV, IVR is indicated by an arrow.
![Figure 5. Superimposition of peptides IIR (yellow), IV (orange), IVR (pink) onto OT (cyan) in the OTR receptor (PDB 7QVM). Residues of OTR in close contact with OT ligand (distance < 5 Å) are coloured in green. Only the side chains of W188, M315 and L316 are shown. Analogues II, IV, IVR were modelled by constraining dihedral angles to adopt the bioactive conformation of OT. The 1H-[1,2,3]triazol-1-yl ring in peptides II, IV, IVR is indicated by an arrow.](/cms/asset/3c27708b-6fb9-48c6-b0e8-493c28022cfe/ienz_a_2254019_f0005_c.jpg)