Figures & data
Figure 1. Examples of 2H-chromene and 7H-furo-chromene-based potential anticancer derivativesCitation51,Citation52,Citation54,Citation55 and newly synthesised chromene series.
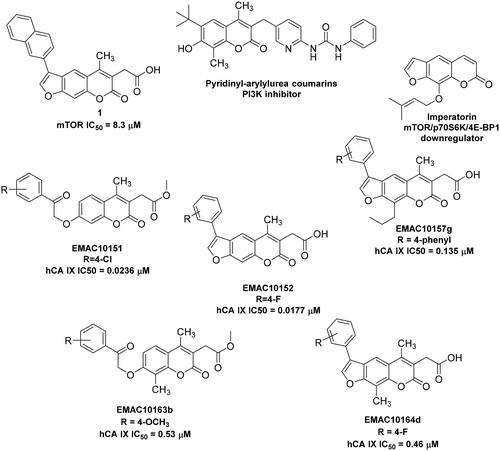
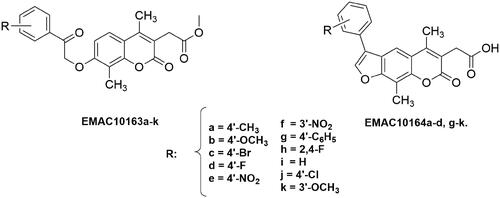
Figure 2. Structure and substitution pattern of 2H-chromene EMAC10163a-k and 7H-furo-chromene EMAC10164a-d, g-k.
Scheme 1. Synthetic pathway to obtain compounds EMAC10163a-k and EMAC10164a-d, g-k. Reagents, and conditions: (i) dimethyl 2-acetylsuccinate, H2SO4 98%; (ii) acetone, K2CO3, α-haloketone, reflux, 6–24 h; (iii) propan-2-ol, NaOH, reflux 2–5 h.
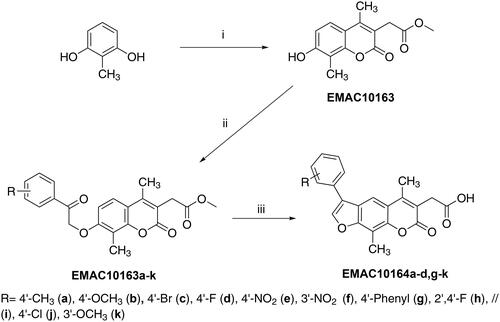
Table 1. Inhibition data towards hCA I, II, IX, and XII of EMAC10163a-k and EMAC10164a-d, g-k.
Figure 3. Representation of the putative binding mode of the EMAC10163a and EMAC10163b compounds obtained by docking experiments in complex with hCA IX. (A) 3D representation of EMAC10163a and its respective interactions with CA IX residues; (B) 2D depiction of interactions; (C) 3D depiction of EMAC10163a and its respective interactions with CA IX residues considering a different orientation of the compound; (D) 2D depiction of interactions; (E) 3D depiction of EMAC10163b and its respective interactions with CA IX residues; (F) 2D depiction of interactions; (G) 3D depiction of EMAC10163b and its respective interactions with CA IX residues considering a different orientation of the compound; (H) 2D depiction of interactions.
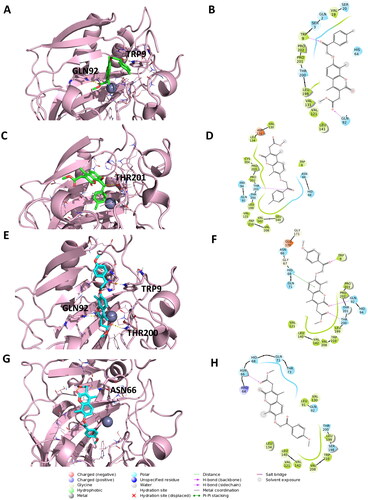
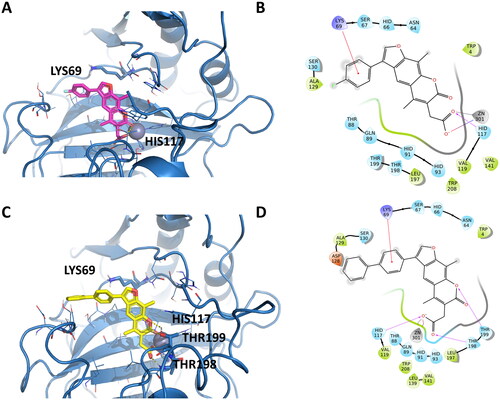
Figure 4. Representation of the putative binding mode of the EMAC10164 series most potent compounds obtained by docking experiments in complex with hCA IX. (A) 3D depiction of EMAC10164d and its respective interactions with CA IX residues; (B) 2D depiction of interactions; (C) 3D depiction of EMAC10164g and its respective interactions with CA IX residues; (D) 2D depiction of interactions.
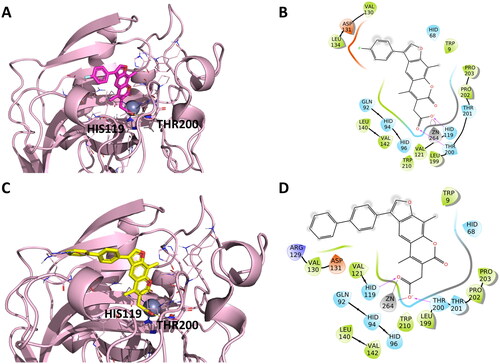
Figure 5. Representation of the putative binding mode of the most potent compounds from EMAC10163-open form series obtained by docking experiments in complex with hCA IX. (A) 3D depiction of EMAC10163a-open-E and its respective interactions with CA IX residues; (B) 2D depiction of interactions; (C) 3D depiction of EMAC10163a-open-Z and its respective interactions with CA IX residues; (D) 2D depiction of interactions; (E) 3D depiction of EMAC10163b-open-E and its respective interactions with CA IX residues; (F) 2D depiction of interactions; (G) 3D depiction of EMAC10163b-open-Z and its respective interactions with CA IX residues; (H) 2D depiction of interactions.
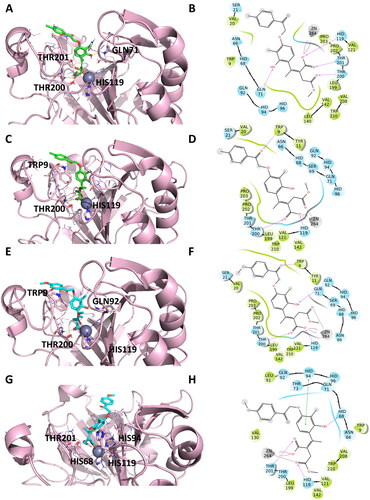
Figure 6. Representation of the putative binding mode of the EMAC10163 series most potent compounds obtained by docking experiments in complex with hCA XII. (A) 3D depiction of EMAC10163a and its respective interactions with CA XII residues; (B) 2D depiction of interactions; (C) 3D depiction of EMAC10163b and its respective interactions with CA XII residues; (D) 2D depiction of interactions; (E) 3D depiction of EMAC10164b and its respective interactions with CA XII residues considering a different orientation of the compound; (F) 2D depiction of interactions.
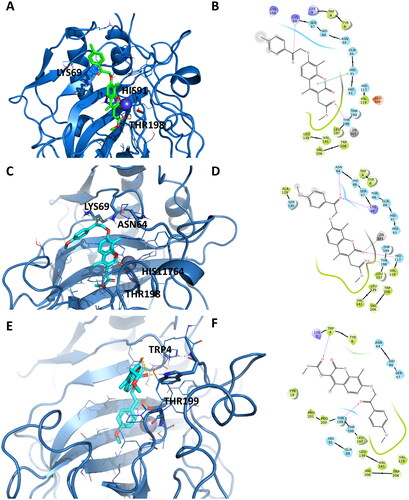
Figure 7. Representation of the putative binding mode of the EMAC10164 series most potent compounds obtained by docking experiments in complex with hCA XII. (A) 3D depiction of EMAC10164d and its respective interactions with CA XII residues; (B) 2D depiction of interactions; (C) 3D depiction of EMAC10164g and its respective interactions with CA XII residues; (D) 2D depiction of interactions.