Figures & data
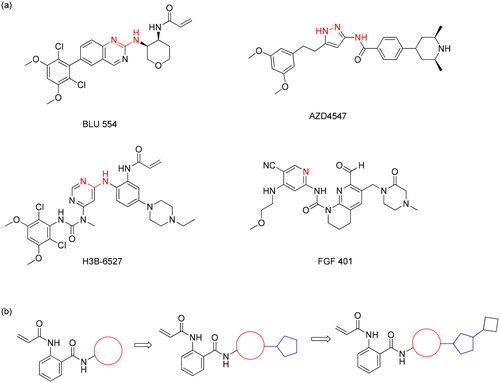
Figure 1. (a) Structures of selective FGFR4 kinase inhibitors. Atoms in red were putatively placed towards hinge region (b) Schematic structure of designed molecules using fragment-assembly strategy.
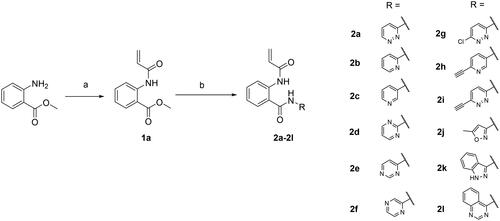
Scheme 1. Reagents and conditions: (a) acryloyl chloride, Et3N, CH2Cl2, rt, 3 h, 44%; (b) arylamine, trimethylaluminum 2 M in toluene, dry toluene, 110 °C, 3 h, 11–35%.
Scheme 2. Reagents and conditions: (a) arylacetylene, (Ph3P)2PdCl2, CuI, dry 1,4-dioxane, rt, overnight, 44–95%; (b) 3-methoxyphenol, picolinic acid, K3PO4, CuI, DMSO, 100 °C, overnight, 52%; (c) 3-methoxyphenylboronic acid, Pd(PPh3)4, K2CO3, THF, water, 130 °C, 1 h, microwave irradiation, 68%; (d) 1a, Al(CH3)3 2 M in toluene, dry toluene, 110 °C, 3 h, 11–31%. (e) i) Pd/C, H2, CH3OH, rt, 3 h; ii) 1a, Al(CH3)3 2M in toluene, dry toluene, 110 °C, 3 h, 5–8%. (f) Pd/C, H2, CH3OH, rt, 3 h, 97%; (g) 3c, Al(CH3)3 2M in toluene, dry toluene, 110 °C, 3 h, 23%.
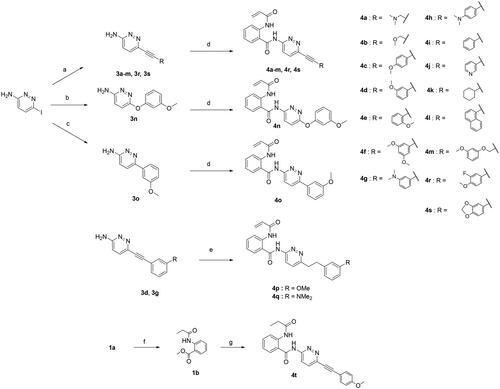
Table 1. In vitro inhibitory activity of compound 24–32, 54–56 against FGFR4 kinase at 1 μM.
Table 2. In vitro inhibitory activity of compounds 4a-4s against FGFR4 kinase at 1 μM.
Table 3. IC50 values of selected compounds (4a, 4d, 4h and 4s).
Table 4. Kinase selectivity of 4c against FGFR subtypes.
Table 5. Metabolic stability of 4c in human and mouse liver microsomes.
Data availability statement
The data supporting the findings of this study are available within the article.

