Figures & data
Table 1. Categories of serotype 2 vaccine-derived poliovirus (VDPV2) events detected since the tOPV-bOPV switch and as of April, 2018, based on our subjective interpretation from limited information [Citation48,Citation49,Citation54,Citation55].
Figure 1. Risks overtime, by serotype and source and defined as the probability of a detected outbreak that triggers an outbreak response (i.e. oSIAs) and based on runs that assumed OPV13 cessation in 2019 [Citation38,Citation40]. (a) All serotypes, triggering events only (including cVDPVs in the event of insufficient OPV intensification). (b) Serotype 1, triggering events and associated outbreaks. (c) Serotype 2, triggering events and associated outbreaks. (d) Serotype 3, triggering events and associated outbreaks.
![Figure 1. Risks overtime, by serotype and source and defined as the probability of a detected outbreak that triggers an outbreak response (i.e. oSIAs) and based on runs that assumed OPV13 cessation in 2019 [Citation38,Citation40]. (a) All serotypes, triggering events only (including cVDPVs in the event of insufficient OPV intensification). (b) Serotype 1, triggering events and associated outbreaks. (c) Serotype 2, triggering events and associated outbreaks. (d) Serotype 3, triggering events and associated outbreaks.](/cms/asset/e698ecfc-ea45-4da2-872f-7681c4730d9e/ierv_a_1506333_f0001_oc.jpg)
Figure 2. Distribution of the number of polio cases after homotypic OPV cessation in the global model base case and analysis of stock-outs (to determine whether a stock-out would occur for a given iteration, we assumed an initial and desired filled stockpile level of 100 million doses with 400 million bulk doses and a one-year filling time.). (a) Serotype 1. (b) Serotype 2. (c) Serotype 3.
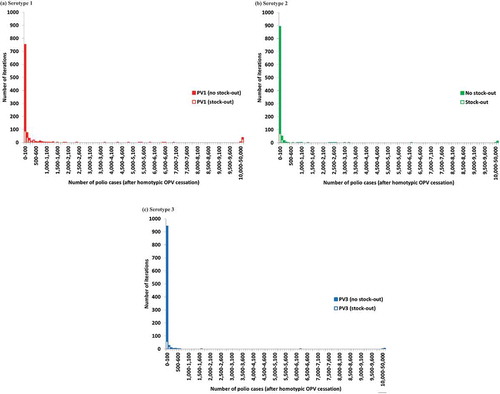
Figure 3. Conditional probability of uncontrolled outbreaks (i.e. more than 50,000 polio cases, which triggers an OPV restart in the global model [Citation38]) as a function of the number of polio cases, based on the results from .
![Figure 3. Conditional probability of uncontrolled outbreaks (i.e. more than 50,000 polio cases, which triggers an OPV restart in the global model [Citation38]) as a function of the number of polio cases, based on the results from Figure 2.](/cms/asset/ae8867ac-f5c4-465e-b3c9-58b43cb5f7c3/ierv_a_1506333_f0003_oc.jpg)
Figure 4. Kinetics of 57 global model iterations that resulted in an OPV restart for different categories of times between homotypic OPV cessation and the initiating event that eventually triggers the OPV restart. (a) Global model base case, with IPV replacing mOPV for oSIAs from 5 years after homotypic OPV cessation (but no other changes in outbreak response strategy). (b) No oSIAs from 5 years after homotypic OPV cessation.
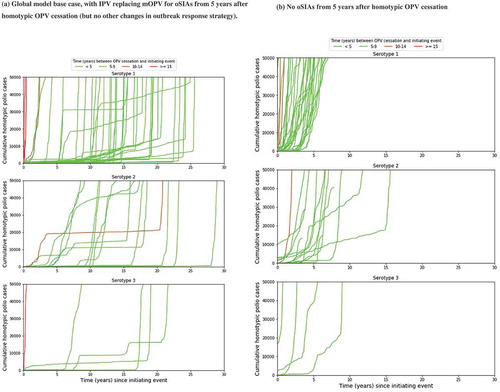
Table 2. Recommended strategies to minimize the risk of an OPV restart and qualitative impacts and resource needs.
