Figures & data
Figure 1. ‘Focus on the Patient’ section to contextualize, highlight, and discuss the impact of the primary findings of our review.
Table 1. Characteristics of clinical trials that evaluated the impact of PHiD-CV.
Table 2. Characteristics of post-marketing effectiveness and impact studies of PHiD-CV. Effectiveness studies compared vaccinated and unvaccinated individuals exposed to the same PHiD-CV vaccination program while impact studies compared the same population before and after the introduction of PHiD-CV in the national immunization program.
Figure 2. Countries in which studies were conducted to assess the efficacy or effectiveness of PHiD-CV against invasive pneumococcal disease (IPD), pneumonia, and acute otitis media (AOM).
aAntimicrobial purchases and tympanostomy tube placement in Finland; AOM-related diagnoses and surgical procedures in Sweden; antimicrobial purchases in Iceland.bStudy 053 was not powered to evaluate AOM end points and the assessment was affected by a randomization error.COMPAS: Clinical Otitis Media and PneumoniA Study; FinIP: Finnish Invasive Pneumococcal disease trial; 7vCRM: 7-valent pneumococcal CRM-conjugate vaccine; 2 + 1/3 + 1/3 + 0, PHiD-CV infant vaccination schedules (two or three primary doses with or without booster dose).
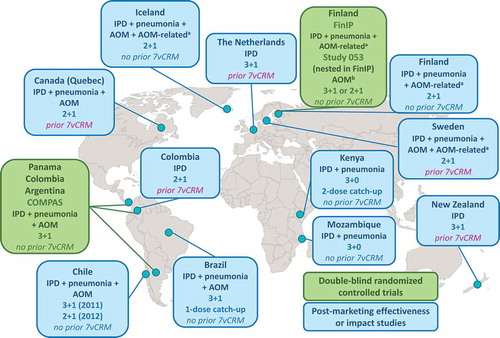
Table 3. Efficacy and effectiveness of PHiD-CV against invasive pneumococcal disease (IPD) in vaccinated or vaccine-eligible infants and young children.
Table 4. Efficacy and effectiveness of PHiD-CV against pneumonia in vaccinated or vaccine-eligible infants and young children in countries where PHiD-CV was the first PCV introduced in the national immunization program.
Table 5. Efficacy and effectiveness of PHiD-CV against acute otitis media (AOM) or AOM-related end points in vaccinated or vaccine-eligible infants and young children in countries where PHiD-CV was the first PCV introduced in the national immunization program.
Figure 3. Impact of PHiD-CV vaccination on nasopharyngeal carriage of vaccine pneumococcal serotypes (VT) and non-vaccine serotypes (NVT) in (a) Panama (COMPAS) [Citation72], (b) Finland (study 053: AOM/carriage study nested in FinIP trial) [Citation11], (c) the Czech Republic (study 014: non-randomized study with age-matched controls) [Citation14], and (d) the Netherlands (study 027: randomized study of children immunized with PHiD-CV or 7vCRM) [Citation15].
*Pneumococcal serotypes included in PHiD-CV (serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F).
![Figure 3. Impact of PHiD-CV vaccination on nasopharyngeal carriage of vaccine pneumococcal serotypes (VT) and non-vaccine serotypes (NVT) in (a) Panama (COMPAS) [Citation72], (b) Finland (study 053: AOM/carriage study nested in FinIP trial) [Citation11], (c) the Czech Republic (study 014: non-randomized study with age-matched controls) [Citation14], and (d) the Netherlands (study 027: randomized study of children immunized with PHiD-CV or 7vCRM) [Citation15].*Pneumococcal serotypes included in PHiD-CV (serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F).](/cms/asset/6fabc8df-5426-4f5b-ae19-96c456019880/ierv_a_1516551_f0003_oc.jpg)
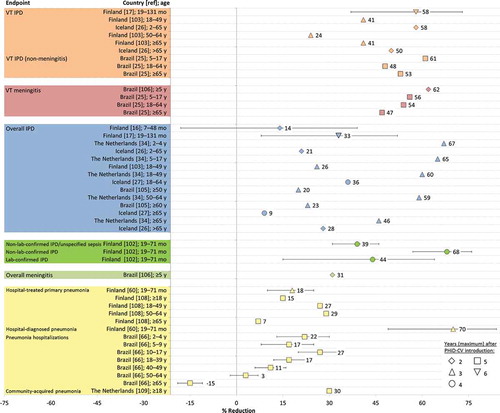
Figure 4. Indirect effects of PHiD-CV on invasive pneumococcal disease (IPD), meningitis, and pneumonia in vaccine-ineligible children and adults estimated from post-marketing impact studies in Finland, the Netherlands, Iceland, and Brazil. Further information on the analyses conducted is provided in Supplementary Table 2.
diag: diagnosed; lab: laboratory; overall IPD: IPD due to any pneumococcal serotype; pneumococcal meningitis: meningitis due to any pneumococcal serotype; VT IPD: IPD due to vaccine serotypes; VT meningitis: meningitis due to vaccine serotypes; mo: months; y: years. Error bars represent 95% confidence intervals.