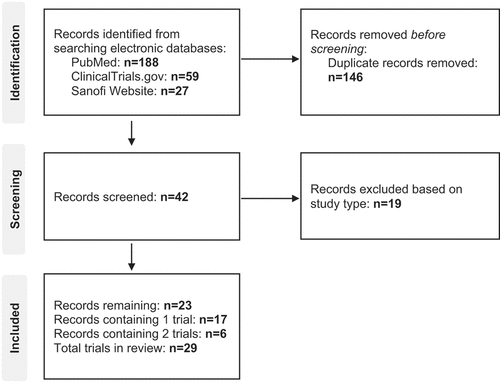
Figures & data
Table 1. Clinical trial characteristics comparing the immunogenicity and safety of primary and booster DT2aP-IPV-Hib-HBV with comparator vaccines.
Table 2. Immune response of DT2aP-IPV-Hib-HBV vs DT3aP-IPV-Hib-HBVfor each antigen post primary or booster vaccination.
Table 3. Clinical trial characteristics of studies without comparator vaccines.