Figures & data
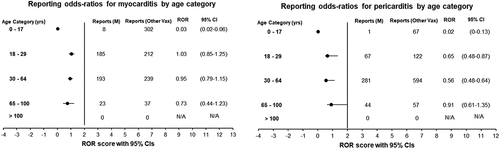
Figure 1. Bar chart depicting the total frequency of adverse events reported to VAERS following mRNA-1273 vaccination classed as medical Dictionary of Regulatory Activities system (MedDRA) organ classes.
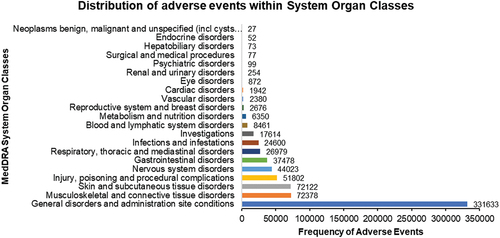
Figure 2. Bubble chart of the overall distribution of AEs within SOCs for three covid-19 vaccines, including mRNA-1273, BNT162b2 and Ad26.COV2.S.
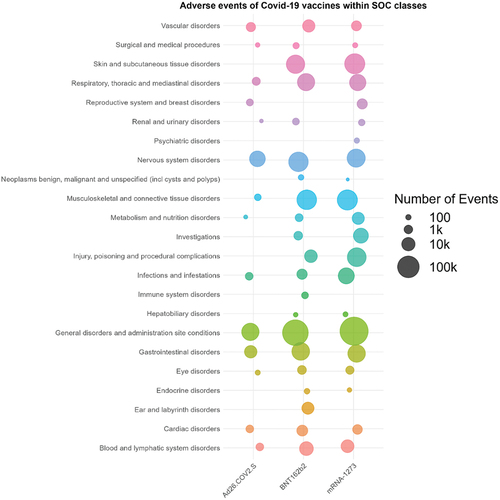
Table 1. Characteristics of VAERS reports received following COVID-19 vaccination between January 1, 2021, and October 27, 2022.
Table 2. Nocebo effects of adverse events associated with mRNA-1273 vaccine.
Figure 3. Forest plots of the ROR and EBGM scores with 95% CIs for the most common mRNA-1273-related adverse events. ROR, reporting odds ratio; EBGM, Empirical Bayesian geometric mean; AE, adverse event; M, mRNA-1273; vax, vaccines; CI, confidence interval.
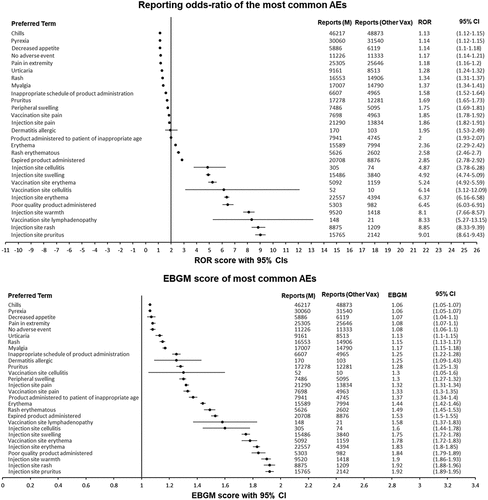
Figure 4. Forest plots of the ROR with 95% CIs for myocarditis and pericarditis by age category. ROR, reporting odds ratio; M, mRNA-1273; vax, vaccines; CI, confidence interval.