Figures & data
Figure 1. Indirect treatment comparison network.
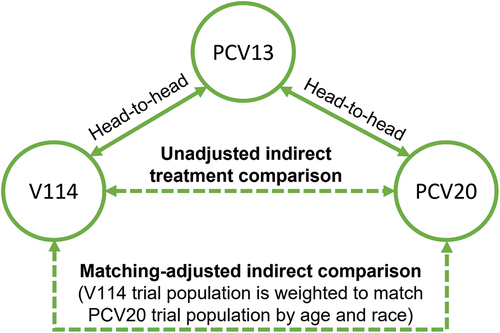
Table 1. Summary description of demographic variables inserted as potential effect modifiers in the MAIC, before and after matching.
Figure 2. Matching-adjusted indirect comparison analysis of the IgG response rate difference for V114 vs PCV20 in healthy infants, ~30 days after completing the third dose (administered at ~6 months of age).
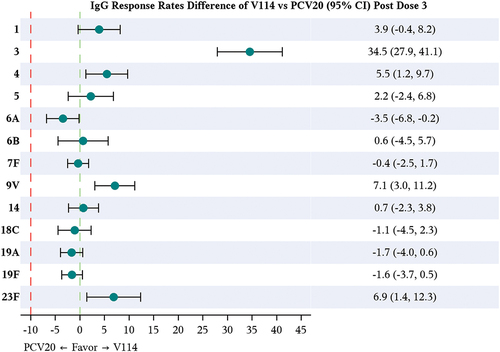
Table 2. Analysis of percentage of infants with a serological response (IgG ≥0.35 µg/mL) 30 days after the third dose (administered at ~6 months of age) – ITC and MAIC of V114 to PCV20.
Figure 3. Matching-adjusted indirect comparison analysis of the IgG GMC ratio for V114 vs PCV20 in healthy infants, ~30 days after completing the third (figure A) and fourth (figure B) dose.
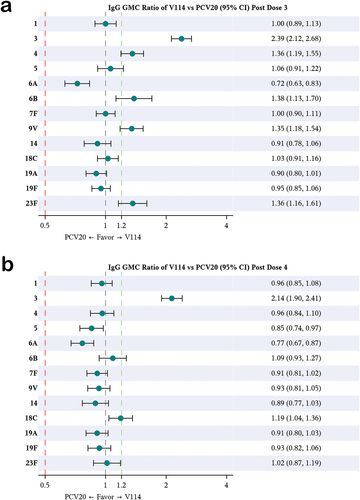
Table 3. Analysis of IgG GMC ratio at 30 days after the third dose (administered at ~6 months of age) – ITC and MAIC of V114 vs PCV20.
Table 4. Analysis of IgG GMC ratio at 30 days after the fourth dose (administered at ~ 12 to 15 months of age) – ITC and MAIC of V114 vs PCV20.
Supplemental Material
Download MS Word (59.7 KB)Data availability statement
Summary/aggregate data from the PCV20 study were used for this analysis and are publicly available online [18, 21-22]; the authors of this study do not own these data. Data from the V114 phase 3 study used in this analysis may be made available to qualified researchers upon reasonable request from the corresponding author and/or Merck & Co., Inc., Rahway, NJ, U.S.A. (Data Access mailbox).
