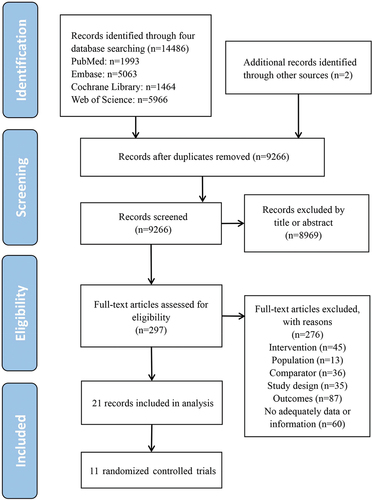
Figures & data
Table 1. Characteristics of the 11 randomized controlled trials.
Figure 2. Network plots. Network plots for persistent infection with HPV 16 (a), Persistent infection with HPV 18 (b), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 16 (c), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 18 (d), Persistent infection with HPV 31 (e), Persistent infection with HPV 33 (f), Persistent infection with HPV 45 (g), Persistent infection with HPV 52 (h), and persistent infection with HPV 58 (i). The area of the circle represents the number of participants. The width of the line represents the number of trials of the two treatments. vHPV, valent human papillomavirus.
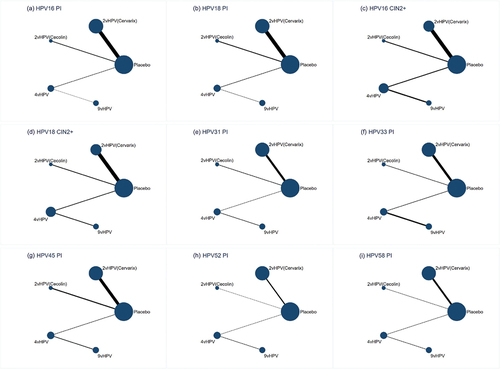
Figure 3. Network meta-analysis. Network meta-analysis for persistent infection with HPV 16 (a), Persistent infection with HPV 18 (b), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 16 (c), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 18 (d), Persistent infection with HPV 31 (e), Persistent infection with HPV 33 (f), Persistent infection with HPV 45 (g), Persistent infection with HPV 52 (h), and persistent infection with HPV 58 (i). Results are column-defined RRs in treatment compared with row-defined RRs in treatment. RR, risk ratio; vHPV, valent human papillomavirus.
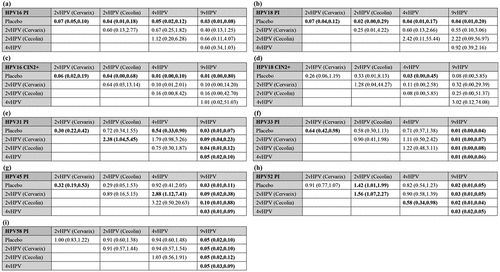
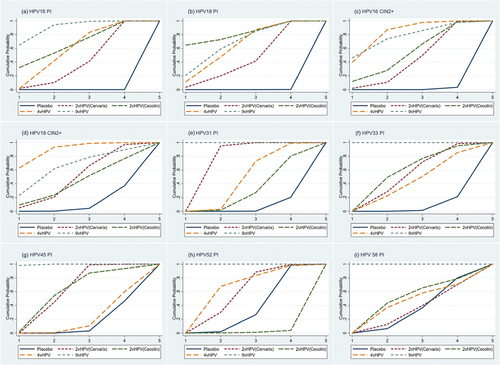
Figure 4. Probability of efficacy rankings for each vaccine. Probability of vaccines’ efficacy for persistent infection with HPV 16 (a), Persistent infection with HPV 18 (b), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 16 (c), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 18 (d), Persistent infection with HPV 31 (e), Persistent infection with HPV 33 (f), Persistent infection with HPV 45 (g), Persistent infection with HPV 52 (h), and persistent infection with HPV 58 (i). The ranking for each vaccine is shown on a cumulative probability plot, with the area under the curve representing the magnitude of the probability. Higher rankings indicate greater efficacy. vHPV, valent human papillomavirus.