Figures & data
Figure 1. (a) Sample selection flowchart in the analysis of vaccine effectiveness against symptomatic SARS-CoV-2 infections. (b) Sample selection flowchart in the analysis of vaccine effectiveness against severe diseases.
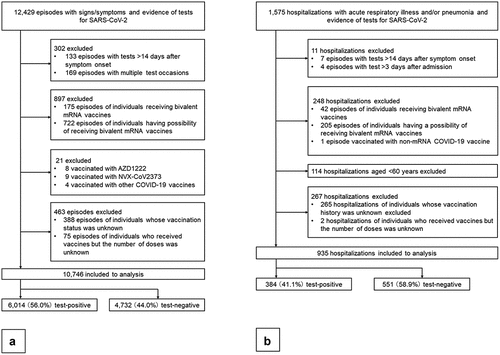
Figure 2. (a) The weekly number of test-positive episodes for SARS-CoV-2 and test-negative episodes for SARS-CoV-2 by vaccination status included in the analysis of vaccine effectiveness against symptomatic SARS-CoV-2 infection. (b) The weekly number of test-positive hospital admissions for SARS-CoV-2 and test-negative hospital admissions for SARS-CoV-2 by vaccination status included in the analysis of vaccine effectiveness against severe diseases.
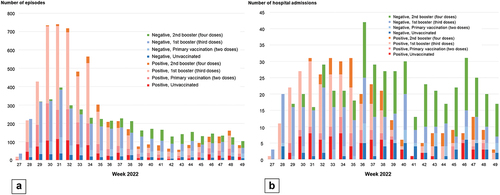
Table 1. Characteristics of individuals included in vaccine effectiveness analysis against symptomatic SARS-CoV-2 infections: VERSUS study, Japan, July 1 to November 30, 2022.
Figure 3. Vaccine effectiveness of monovalent mRNA COVID-19 vaccines against symptomatic SARS-CoV-2 infections among individuals aged 16 to 59 years and aged 60 years, VERSUS study, Japan, between July 1 and November 30, 2022. The analysis included test-positive cases with signs or symptoms that tested positive for SARS-CoV-2 and test-negative controls with signs or symptoms that tested negative for SARS-CoV-2. Vaccine effectiveness was adjusted for age, sex, underlying medical conditions, calendar week of test, healthcare professional status, prior SARS-CoV-2 infection history, and medical facilities. The vaccination status was classified into four based on the number of vaccine doses received before symptom onset and number of days between the last vaccination date and symptom onset: unvaccinated, individuals had received no vaccine dose before symptom onset; primary vaccination, individuals had received the second dose
14 days before symptom onset; 1st booster vaccination, individuals had received the third dose
14 days before symptom onset; and 2nd booster vaccination, individuals had received the fourth dose
14 days before symptom onset. Completion of vaccination was defined as 14 days after receiving the last vaccine. When evaluating vaccine effectiveness separated by days after vaccination, the number of days is shown from completion of vaccination.
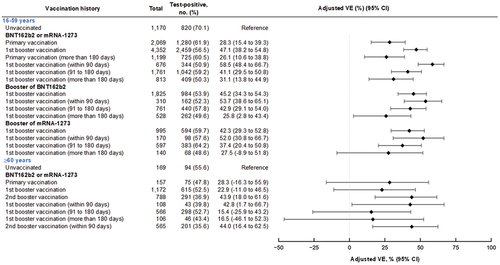
Figure 4. Relative vaccine effectiveness of monovalent mRNA COVID-19 booster vaccination, compared with the primary vaccination at more than 180 days after the completion of vaccination against symptomatic SARS-CoV-2 infections among individuals aged 16 to 59 years and 60 years, VERSUS study, Japan, between July 1 and November 30, 2022. The analysis included test-positive cases with signs or symptoms that tested positive for SARS-CoV-2 and test-negative controls with signs or symptoms that tested negative for SARS-CoV-2. Relative vaccine effectiveness was adjusted for age, sex, underlying medical conditions, calendar week of test, healthcare professional status, prior SARS-CoV-2 infection history, and medical facilities. The vaccination status was classified into four based on the number of vaccine doses received before symptom onset and number of days between the last vaccination date and symptom onset: unvaccinated, individuals had received no vaccine dose before symptom onset; primary vaccination, individuals had received the second dose
14 days before symptom onset; 1st booster vaccination, individuals had received the third dose
14 days before symptom onset; and 2nd booster vaccination, individuals had received the fourth dose
14 days before symptom onset. Completion of vaccination was defined as 14 days after receiving the last vaccine. When evaluating vaccine effectiveness separated by days after vaccination, the number of days is shown from completion of vaccination. Abbreviation: VE, vaccine effectiveness.
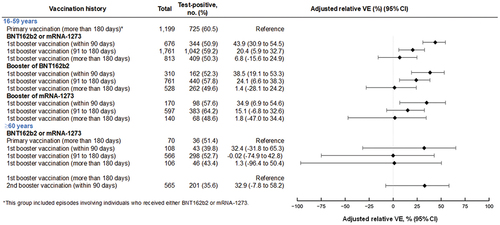
Table 2. Characteristics of individuals aged 60 years included in vaccine effectiveness analysis against severe COVID-19: VERSUS study, Japan, July 1 to November 30, 2022.
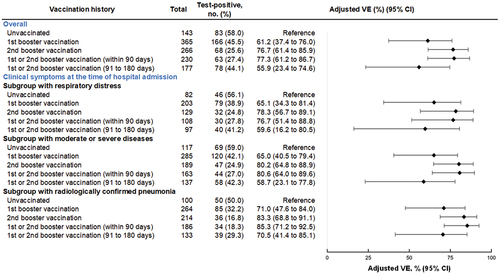
Figure 5. Vaccine effectiveness of monovalent mRNA COVID-19 vaccines against severe diseases among individuals aged 60 years, VERSUS study, Japan, between July 1 and November 30, 2022. The analysis included test-positive cases with signs or symptoms that tested positive for SARS-CoV-2, and test-negative controls with signs or symptoms that tested negative for SARS-CoV-2. Vaccine effectiveness was adjusted for age, sex, underlying medical conditions, calendar week of admission, prior SARS-CoV-2 infection history, and medical facilities. The vaccination status was classified into four based on the number of vaccine doses received before symptom onset and number of days between the last vaccination date and symptom onset: unvaccinated, individuals had received no vaccine dose before symptom onset; primary vaccination, individuals had received the second dose
14 days before symptom onset; 1st booster vaccination, individuals had received the third dose
14 days before symptom onset; and 2nd booster vaccination, individuals had received the fourth dose
14 days before symptom onset. Completion of vaccination was defined as 14 days after receiving the last vaccine. When evaluating vaccine effectiveness separated by days after vaccination, the number of days is shown from completion of vaccination. The following subgroup analyses were conducted: a subgroup with respiratory distress at the time of admission by limiting analysis to individuals with hypoxia (oxygen saturation<90%), oxygen administration, or tachypnea (respiratory rate≥30/minute); a subgroup with moderate or severe diseases at the time of admission defined CURB-65≥2; and a subgroup with radiologically confirmed pneumonia at the time of admission.