Figures & data
Figure 1. Flow diagram of the model for cancers associated with human papillomavirus infection.
Figure 2. Forecasted incidence of nonavalent human papillomavirus vaccine-strain-attributable (a) cervical cancer and (b) all other anogenital plus head & neck cancers in the Netherlands with strategies using a bivalent or nonavalent human papillomavirus vaccine for individuals ≥9 years of age before sexual debut, with or without catch-up vaccination programs for individuals ≤26 years of age.
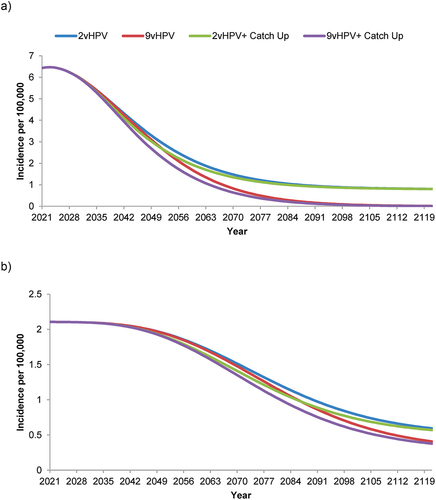
Figure 3. Forecasted incidence of nonavalent human papillomavirus vaccine-strain-attributable anogenital warts in the Netherlands with strategies using a bivalent or nonavalent human papillomavirus vaccine for individuals ≥9 years of age before sexual debut, with or without catch-up vaccination programs for individuals ≤26 years of age.
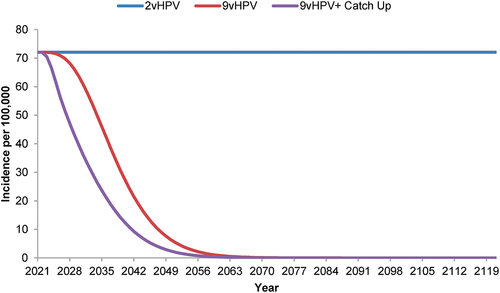
Figure 4. Forecasted incidence of nonavalent human papillomavirus vaccine-strain-attributable (a) juvenile-onset and (b) adult-onset recurrent respiratory papillomatosis in the Netherlands with strategies using a bivalent or nonavalent human papillomavirus vaccine for individuals ≥9 years of age before sexual debut, with or without catch-up vaccination programs for individuals.
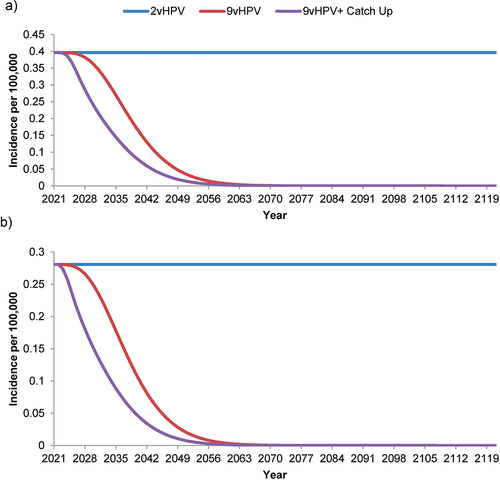
Table 1. Nonavalent human papillomavirus vaccine-strain-attributable disease cases and deaths in the Netherlands over 100 years with use of 9vHPV versus 2vHPV, with and without catch-up vaccination.
Table 2. Cost-effectiveness outcomes of four base-case human papillomavirus vaccination strategies in the Netherlands over 100 years.
Figure 5. Deterministic sensitivity analysis of the incremental cost-effectiveness ratios of 9vHPV versus 2vHPV human papillomavirus vaccination strategies for the Netherlands over 100 years.
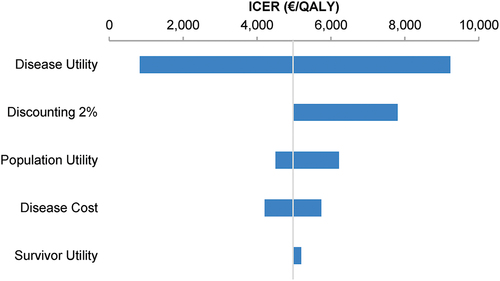
Table 3. Cost-effectiveness outcomes of four human papillomavirus vaccination strategies in the Netherlands over 100 years in scenarios of increased vaccination coverage or cross-protection against additional viral strains.
