Figures & data
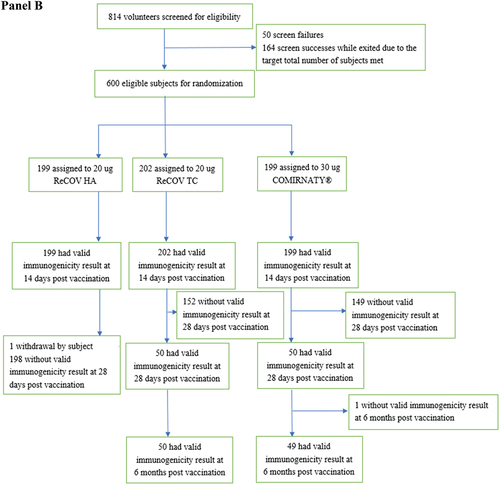
Figure 1. Flow diagram of study-1 (panel A) and study-2 (panel B).
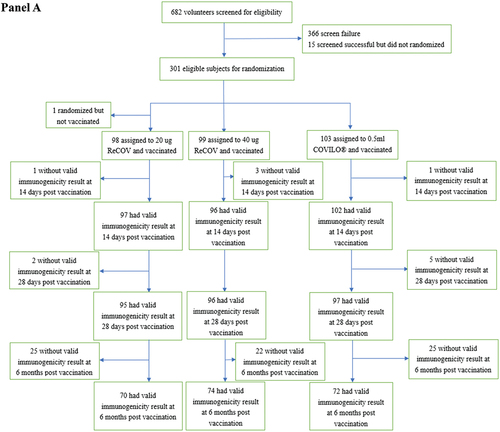
Table 1. Subjects demographic characteristics of study-1 and study-2.
Figure 2. GMTs of live-virus neutralizing antibody against SARS-CoV-2 prototype in study-1 (panel A) and study-2 (panel B).
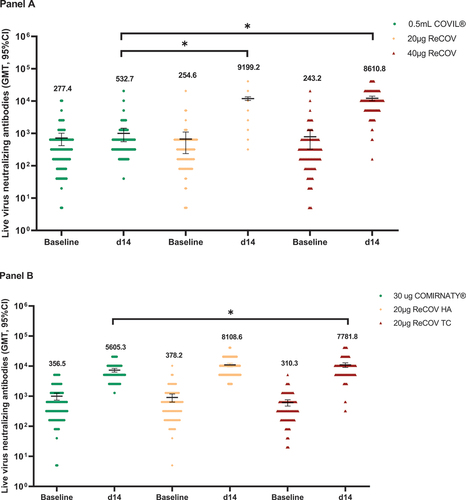
Figure 3. GMTs of pseudovirus neutralizing antibody against SARS-CoV-2 prototype and omicron variants in study-1 (panel A) and study-2 (panel B).
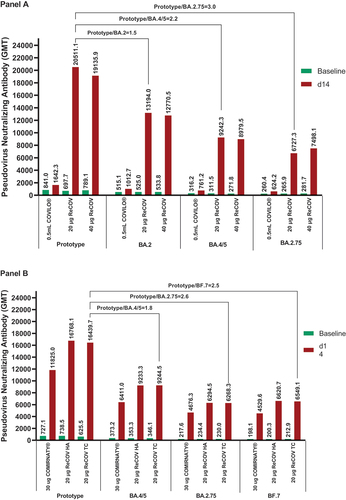
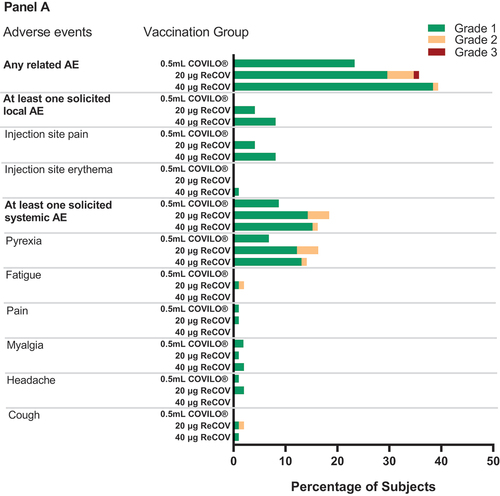
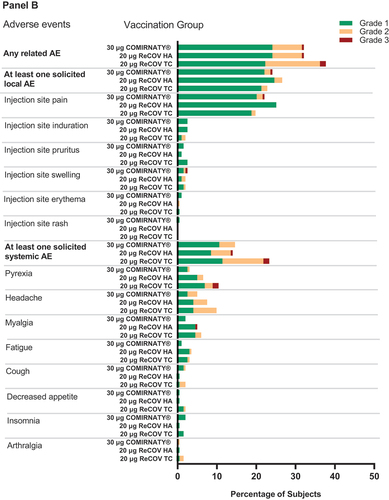
Figure 4. Frequency of any related adverse events, solicited local and systemic adverse eveIn study-1 (panel A) and study-2 (panel B).