Figures & data
Table I. Clinical and obstetrical characteristics of patients with and without intra-amniotic infection (IAI).
Figure 1. Median amniotic fluid concentration of SP-B was significantly higher in patients with intra-amniotic infection (IAI) than those without IAI (IAI: median 40.2 ng/mL, range 0–809 ng/mL vs. without IAI: median 6.2 ng/mL, range 0–1035 ng/mL; p = 0.03). Similar result was observed after adjusting the amniotic fluid SP-B concentration according to the total protein concentration (SP-B/ total protein, IAI: median 7.19 ng/mg, range 0–237.5 ng/mg vs. without IAI: median 0.94 ng/mg, range 0–79.5 ng/mg; p = 0.03). *p < 0.05.
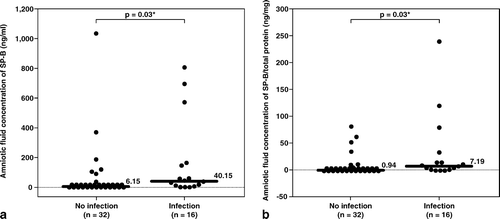
Table II. Amniotic fluid concentrations of SP-A and SP-D in patients with and without intra-amniotic infection (IAI).
Table III. Clinical and obstetrical characteristics of patients who had and who had not received antenatal corticosteroids within 7 days prior to amniocentesis, classified by the presence or absence of intra-amniotic infection (IAI).
Figure 2. Among patients who had received antenatal corticosteroids within 7 days prior to amniocentesis, the median amniotic fluid concentration of SP-B was significantly higher in patients with intra-amniotic infection (IAI) than those without IAI (IAI: median 148 ng/mL, range 37.3–809 ng/mL vs. without IAI: median 7.2 ng/mL, range 0–1035 ng/mL; p = 0.005). Similar result was observed after adjusting the amniotic fluid SP-B concentration according to the total protein concentration (SP-B/ total protein, IAI: median 14.1 ng/mg, range 4.3–237.5 ng/mg vs. without IAI: median 1.45 ng/mg, range 0–79.5 ng/mg; p = 0.003). *p < 0.05.
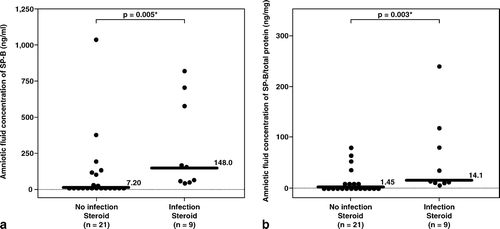
Figure 3. Among patients who had not received antenatal corticosteroids within 7 days prior to amniocentesis, there was no significant difference in the median amniotic fluid concentration of SP-B between patients with and without intra-amniotic infection (IAI) (IAI: median 4 ng/mL, range 0–31.4 ng/mL vs. without IAI: median 3.4 ng/mL, range 0–37 ng/mL; p = 0.8). Similar result was observed after adjusting the amniotic fluid SP-B concentration according to the total protein concentration (SP-B/total protein, IAI: median 0.55 ng/mg, range 0–6.9 ng/mg vs. without IAI: median 0.59 ng/mg, range 0–3.3 ng/mg; p = 0.9).
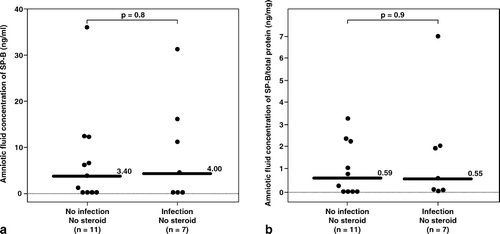
Table IV. Amniotic fluid concentrations of SP-A and SP-D in patients who had and who had not received antenatal corticosteroids within 7 days prior to amniocentesis.