Figures & data
Figure 1. (a) Risk of sPTB before 34 weeks based on fFN concentration. This figure does not present data for all thresholds reported because of the substantial heterogeneity observed. The figure presents thresholds that facilitate an assessment of the trend in sPTB based on the incremental fFN thresholds reported. Outcomes for the other thresholds, not shown here, are reported in . Data presented here are reported for the overall cohort enrolled in each study; subgroup data are not presented. fFN: fetal fibronectin; sPTB: spontaneous preterm birth. (b). Risk of sPTB before 37 weeks based on fFN concentration. This figure does not present data for all thresholds reported because of the substantial heterogeneity observed. The figure presents thresholds that facilitate an assessment of the trend in sPTB based on the incremental fFN thresholds reported. Outcomes for the other thresholds, not shown here, are reported in . Data presented here are reported for the overall cohort enrolled in each study; subgroup data are not presented. NuMoM2b collected fFN specimens at three timepoints; data are presented for the final follow-up visit (22–30 weeks). fFN: fetal fibronectin; sPTB: spontaneous preterm birth.
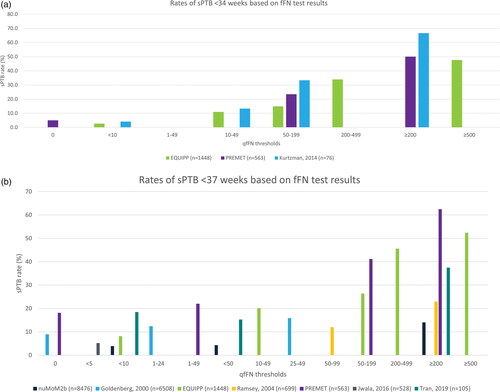
Table 1. Summary of included studies and sPTB rates by fFN levels.
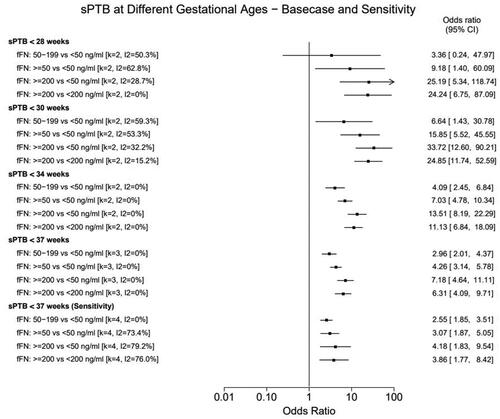
Figure 2. Meta-analysis results: sPTB risk for women with high vs low fFN concentrations. Forest plot showing the results of the random-effects meta-analyses. Results show the likelihood (odds ratio) that patients with higher fFN concentrations will experience sPTB relative to patients with lower fFN concentrations. The higher the odds ratio, the greater risk of sPTB among patients with the higher fFN concentrations relative to the reference group. The 28-week analysis pooled data reported by PREMET and Tran 2019. The 30- and 34-week analysis pooled data reported by EQUIPP and PREMET. The base case 37-week analyses pooled data reported EQUIPP, PREMET and Tran 2019; the sensitivity analysis also adds data from the nuMoM2b study. CI: confidence interval; fFN: fetal fibronectin; sPTB: spontaneous preterm birth
Supplemental Material
Download MS Word (77.7 KB)Data availability statement
Study protocol available upon request. The data used in the analyses are presented in .

