Figures & data
Figure 1. PBOX-15 inhibits proliferation of human CRC cell lines. (A and B) CRC cell lines (1 × 104 / 0.33 cm2 plate) were incubated with increasing concentrations of PBOX-15 (from 0.001 to 5 µM) for 24 and 48 hours. Cell proliferation was evaluated by measuring BrdU incorporation during DNA synthesis through a colorimetric ELISA assay. The results are shown as the mean ± SD from triplicate cultures performed at least 3 times. (**p <0.01, ANOVA compared to the untreated control cells).
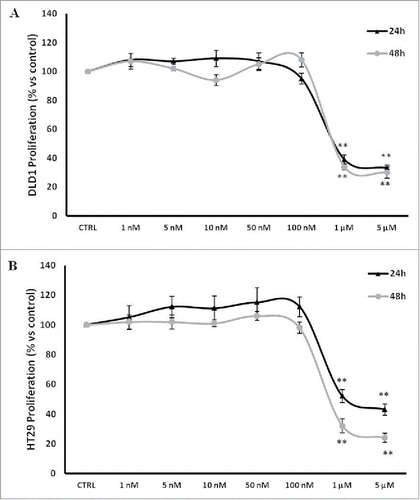
Figure 2. PBOX-15 induces a significant cell cycle arrest in the G2/M phase of DLD-1 and HT29. The percentage of cells was determined by FACS following PtdIns incorporation. The histograms report the percentage of cells in G0/G1, S and G2/M phases after treatment of DLD1 (A) or HT29 (B) cell lines with PBOX-15 (0.1 and 1 µM) for 24 and 48 hours. Each value is the mean ± SD of 3 separate experiments performed in duplicates (***p <0.005, ANOVA).
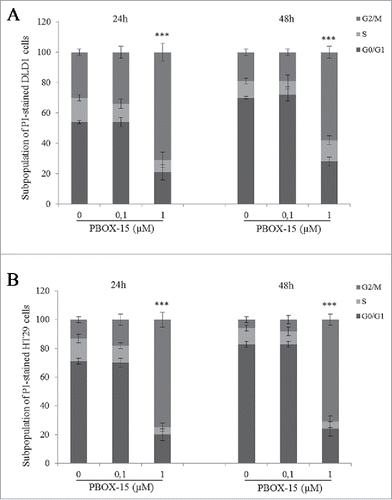
Figure 3. In vitro assessment of apoptosis in DLD-1 cells. (A) DNA-fragmentation assay in DLD-1 cells.DNA was prepared from CRC untreated or treated respectively for 48 h with PBOX-15 (1 µM) and analyzed in a 1.6% agarose gel. Lane M is the 100-bp DNA ladder. (B) Annexin V-FITC/PI staining was used to corroborate the induction of apoptosis. The induction of apoptosis was determined by flow cytometric analysis of Annexin V-FITC and PtdIns-staining. Cells in the lower right quadrant indicate Annexin V-positive, early apoptotic cells. The cells in the upper right quadrant indicate Annexin V-positive/PI-positive, late apoptotic cells. (C) The histograms represents total apoptosis (% of Annexin V+ Annexin V/PtdIns positive cells) in PBOX15-treated cells compared with the untreated control.
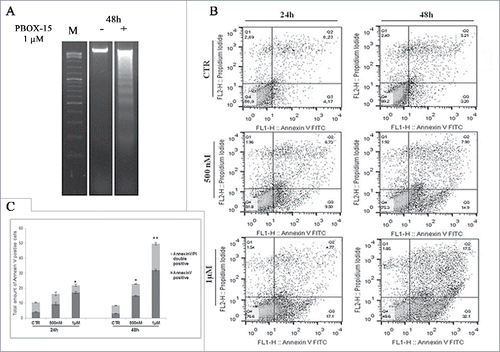
Figure 4. PBOX-15 affects both the apoptotic cascade and MAPKs pathway. (A and B) Lysates from DLD-1 cells, untreated (−) or treated with PBOX-15 for the indicated time points at the concentrations of 100 nM and 1 µM, were immunoblotted with anti-caspase-9, anti-caspase-3, anti-PARP (total and cleaved forms) and anti-pH2AX antibodies (panel A), and with anti-ERK or anti-pERK antibodies (panel B). To confirm equal loading the filters were stripped and reprobed with anti-GAPDH antibody. (C) Lysates from DLD-1 cells untreated (−) or treated (+) with PBOX-15 (1 µM) for the indicated incubation times, were immunoblotted with anti-JNK and anti-pJNK antibodies. Blots shown are representative of at least 3 independent experiments.
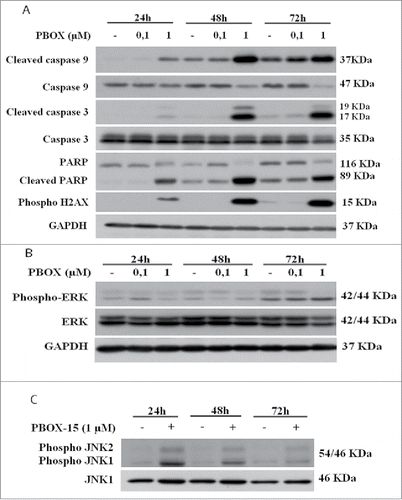
Figure 5. Anti-proliferative effect of PBOX-15, Oxaliplatin and 5-FU in DLD-1 cells. (A) DLD-1 cells were incubated with increasing concentrations of PBOX-15 (ranging from 0.005 µM to 0.3 µM), Oxaliplatin (ranging from 0.08 µM to 10 µM) or 5-FU (ranging from 3 µM to 100 µM) for 72 hours. The dose-effect curve was performed using the CalcuSyn software and it showed the dose of the drug vs the fraction of the cells affected/killed by PBOX15, Oxaliplatin or 5-FU used alone. A representative experiment (carried out at least twice) is reported. (B) DLD-1cells were treated for 72 hours with Oxaliplatin (0.08-10 µM) or PBOX-15 in combination with Oxaliplatin (1:10 molar ratio) at the concentrations indicated in the table. (C) DLD-1 cells were treated for 72 hours with 5-FU (3-100 µM) or PBOX-15 in combination with 5-FU (1:100 molar ratio) at the concentrations indicated in the table. Cell proliferation was evaluated by measuring BrdU incorporation during DNA synthesis through a colorimetric ELISA assay. The dose-effect curve was done using the CalcuSyn software and a representative experiment (carried out at least twice) is reported.
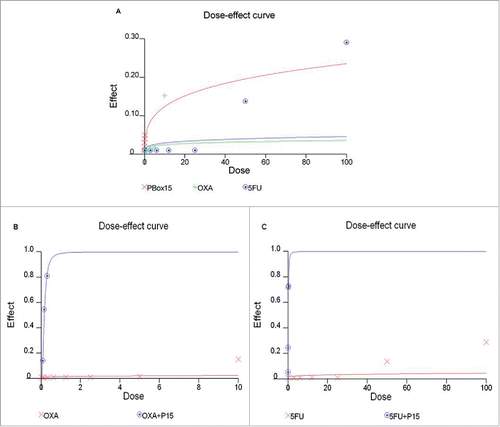
Table 1. Combination index (CI), Fraction affected (FA) and Dose Reduction Index (DRI) for PBOX-15 and OXA/5-Fluorouracil in DLD-1 cells.
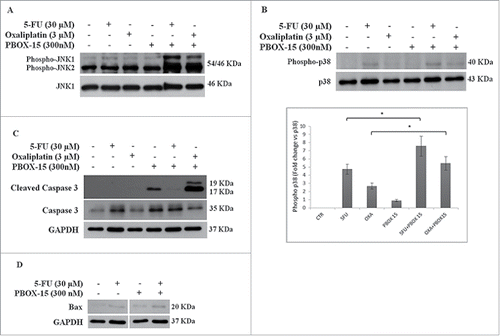
Figure 6. Synergistic effect may possibly involve MAPK pathways. (A, B and C) Lysates from DLD-1 cells, untreated (−) or treated (+) with Oxaliplatin (3 μM), 5-Fluorouracil (30 μM) and PBOX-15 (300 nM) alone or in combination for 48 h (panel A) or 72 h (panels B and C) were immunoblotted with anti-JNK (total and phosphorylated forms in panel A), with anti-p38 (total and phosphorylated forms in panel B), with anti-caspase 3 (total and cleaved forms in panel C) antibodies. In B lower panel, the histograms shown represent the densitometric analyses of phospho-p38 expressed as fold change of the total p38 amount. Each value is the mean ± SD of 3 independent experiments (*p <0.05, ANOVA). (D) Lysates from DLD-1 cells, untreated or treated with 5-FU (30 μM) and PBOX-15 (300 nM) alone or in combination for 72 h, were immunoblotted with anti-Bax antibody. To confirm equal loading, the filters were stripped and reprobed with anti-GAPDH antibody. Blots shown are representative of at least 3 independent experiments with similar results.