Figures & data
Table 1. Efficacies and transition probabilities
Table 2. Rates of second-line treatment in the study
Table 3. Incidence of AE (grade 3/4) related to costs collected from the FIRE-3.
Table 4. Results of the cost-effectiveness analysis
Figure 1. Markov model for metastatic colorectal cancer. According to the FIRE-3 profile, 4 groups were analyzed: group 1, patients with KRAS testing treated with Cmab and FOLFIRI; group 2, patients with RAS testing treated with Cmab and FOLFIRI; group 3, patients with KRAS testing treated with Bmab and FOLFIRI; group 4, patients with RAS testing treated with Bmab and FOLFIRI. A Markov model comprising 3 health states (progression-free survival, progressive disease and death) was built. mCRC,metastatic colorectal cancer; Cmab,cetuximab;Bmab,bevacizumab; PD, progression disease; PFS, progression-free survival; AE, adverse event.
Figure 2. Cost-effectiveness pictured with 4 groups. Four groups were analyzed: group 1, patients with KRAS testing treated with Cmab and FOLFIRI; group 2, patients with RAS testing treated with Cmab and FOLFIRI; group 3, patients with KRAS testing treated with Bmab and FOLFIRI; group 4, patients with RAS testing treated with Bmab and FOLFIRI.The KRAS-Cmab strategy was dominated by the other 3 groups. mCRC, metastatic colorectal cancer; Cmab, cetuximab;Bmab, bevacizumab; QALM, quality-adjusted life-months.
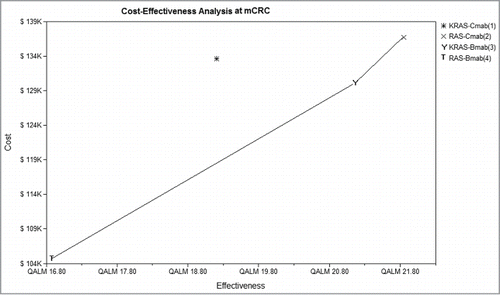
Figure 3. Cost-effectiveness scatterplot of 4 groups. Four groups were analyzed: group 1, patients with KRAS testing treated with Cmab and FOLFIRI; group 2, patients with RAS testing treated with Cmab and FOLFIRI; group 3, patients with KRAS testing treated with Bmab and FOLFIRI; group 4, patients with RAS testing treated with Bmab and FOLFIRI. Cost-effectiveness distribution of 4 groups. CE, Cost-effectiveness; Cmab, cetuximab;Bmab, bevacizumab.
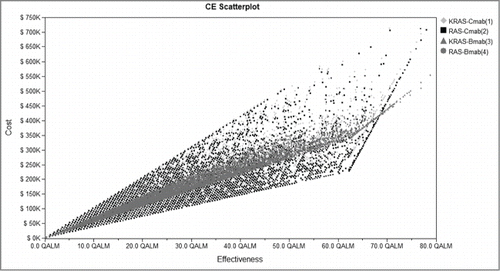
Figure 4. Tornado diagram of one-way sensitivity analysis. Tornado diagram summarized the results of one-way sensitivity analysis to identify model variables associated with the 4 strategies in the treatment of mCRC. The influential factors were listed descending with the variation of value. mCRC, metastatic colorectal cancer; AE, adverse event.
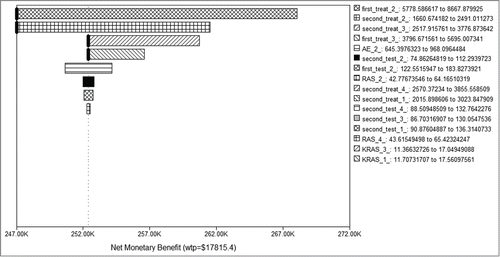
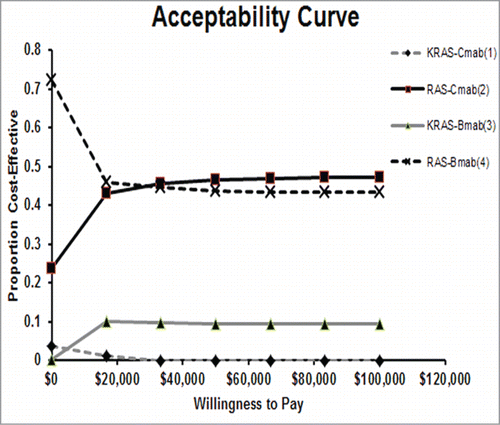
Figure 5. Probabilistic sensitivity analysis (acceptability frontier). The cost-effectiveness acceptability frontier shows the probabilistic sensitivity analysis -based probability of strategies being cost-effective in 4 strategies. For different willingness to pay thresholds, different strategies are optimal. Cmab,cetuximab;Bmab,bevacizumab.