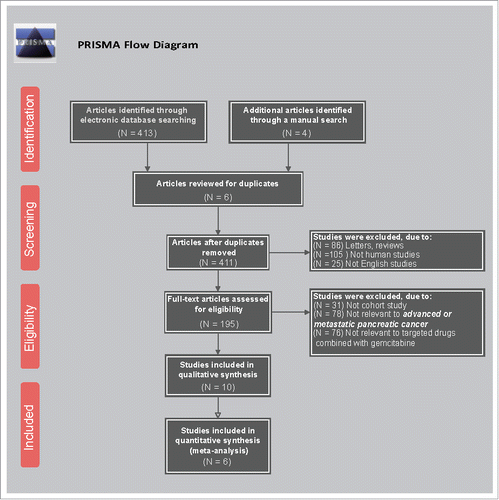
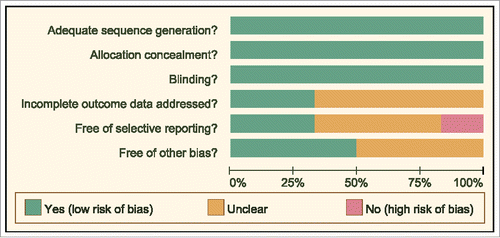
Figures & data
Table 1. Estimated OR and 95%CI of pairwise meta-analysis for hematologic toxicity events in advanced or metastatic pancreatic cancer patients.
Table 2. Estimated OR and 95%CI of pairwise meta-analysis for non-hematologic toxicity events in advanced or metastatic pancreatic cancer patients.
Figure 3. The network evidence graphs of anemia, neutropenia, thrombocytopenia, rash, diarrhea and stomatitis (all grades, grade ≥ 3) (Gemcitabine + Placebo is taken as the reference for all targeted therapies; The size of the circle represents the sample size. The larger the circle, the larger the sample size is).
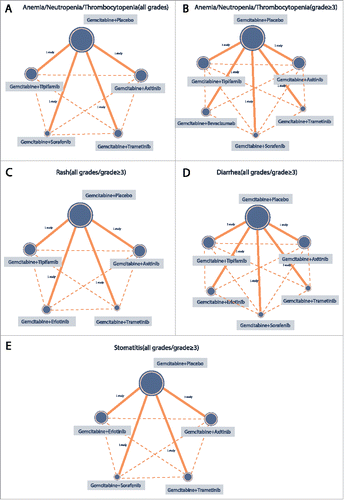
Table 3. OR and 95%CI of six treatment modalities of six hematologic endpoint outcomes.
Table 4. OR and 95%CI of six treatment modalities of six non-hematologic endpoint outcomes.
Table 5. SUCRA values of seven treatment modalities under twelve endpoint outcomes.
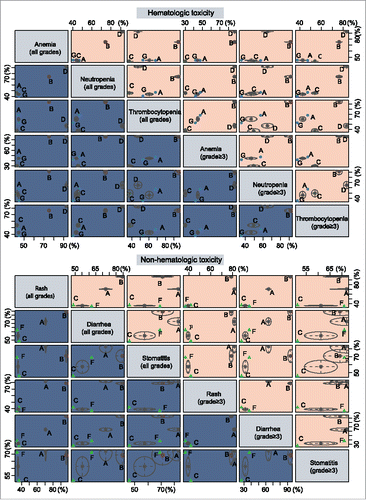
Figure 4. Cluster analyses of anemia, neutropenia, thrombocytopenia, rash, diarrhea and stomatitis. (The 6 outcome indexes are considered, and the advantages and disadvantages of each intervention are compared; In each small square, the intervention measures in the upper right corner are “superior”, and the intervention measures in the lower left corner are “inferior”.).