Figures & data
Figure 1. Characterization of mesenchymal cells derived from human Alveolar Mucosa. Typical data of one AMC culture is presented. Analysis of all investigated AMC has shown no significant deviations for cultures obtained from ten different patients. (A) Anatomical regions of human gingiva. The sources of Alveolar Mucosa (AMC) Attached Gingiva (AGC) and free gingiva cells are indicated. (B) Protein profiling of AMC. FACScan analysis data of different protein markers expression in AMC at early passages (2–3p). (C) Expression of positive mesenchymal markers in AMC*. (D) Immunofluorescence analysis of mesenchymal markers confirming morphology of investigated AMC, cell nuclei stained with DAPI (Bars 25 μm)*. * - Data of negative (unexpressed in AMC) markers is available in Suppl.1.
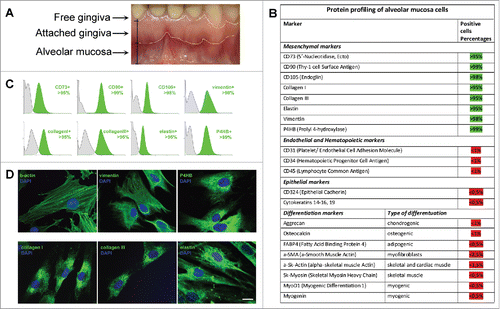
Figure 2. Invariability of AMC morphology and karyotype stability at passages 1 and 10. Typical data of one of ten AMC cultures is presented; similar results have been obtained for other cultures. (A) Phase contrast microscopy of AMC culture at passages 1 and 10 (Bars 50 μm). (B) Immunofluorescence analysis of f-actin bundles (TRITC-phalloidin-red) of AMC at passages 1 and 10, cell nuclei stained with DAPI (Bars 50 μm). (C) Immunofluorescence analysis of morphology of interphase nuclei (stained with DAPI) of AMC at passages 1 and 10 (Bars 50 μm). (D) AMC's karyotyping at passages 1 and 10. G-band analysis of metaphase chromosomes.
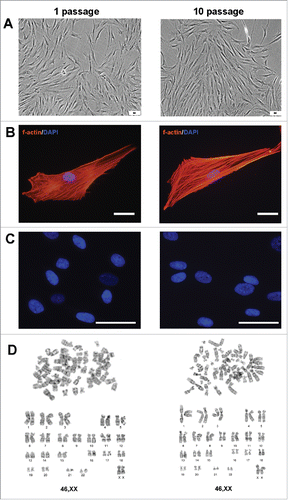
Figure 3. Abilities of AMC to differentiate into chondro-, osteo- and adipogenic directions. Immunofluorescence analysis of chondro- (aggrecan), osteo- (osteocalcin) and adipogenic (FABP4) markers under normal (upper panel) and differentiation inducting (lower panel) conditions, cell nuclei stained with DAPI (Bars 50 μm). Typical data of one of ten AMC cultures is presented; all ten tested AMC cultures were able to differentiate into three lineages.
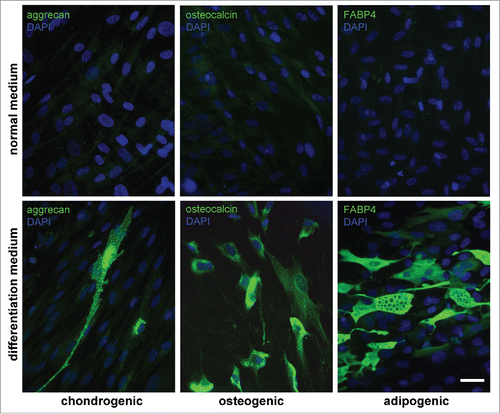
Figure 4. Ability of AMC to TGBβ1-induced myofibroblast differentiation. (A) Immunofluorescence analysis of α-smooth muscle actin (αSMA) (green) of AMC after 72 h of permanent TGFβ1 treatment and 5 passages of permanent TGFβ1 treatment, cell nuclei stained with DAPI (Bars 25 μm). Typical immunofluorescence staining of one of ten AMC cultures is presented. (B) FACScan analysis of α-smooth muscle actin (αSMA) (green) after 72 h of permanent TGFβ1 treatment and 5 passages of permanent TGFβ1 treatment of AMC culture presented in section 4A. (C) Percentage of αSMA-positive cells after 72 h of permanent TGFβ1 treatment and 5 passages of permanent during passaging TGFβ1 treatment. Average data for ten AMC cultures is presented (Mean ± SD).
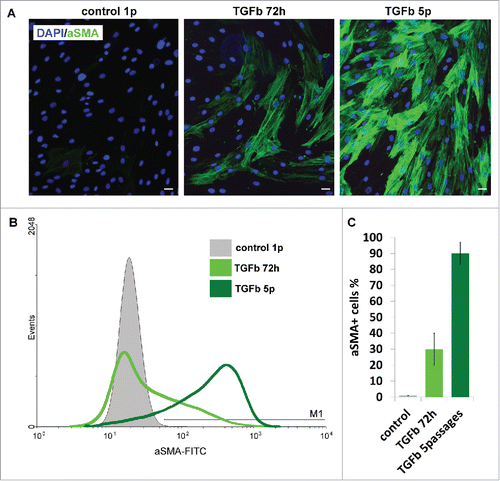
Figure 5. Microscopy analysis of myogenic proteins in AMC differentiated in myogenic direction. Typical data of one AMC culture is presented. All investigated AMC cultures obtained from ten different patients were able to differentiate in myogenic direction. (A) AMC derived myotubes phase contrast microscopy (Bars 25 μm). (B) AMC derived myotubes DIC microscopy. Suppl.2 myotube contraction video. (C) Immunofluorescence analysis of skeletal myosinandactin (red), myogenic transcriptional factors myogenin and MyoD1 (green), cell nuclei stained with DAPI (Bars 25 μm).
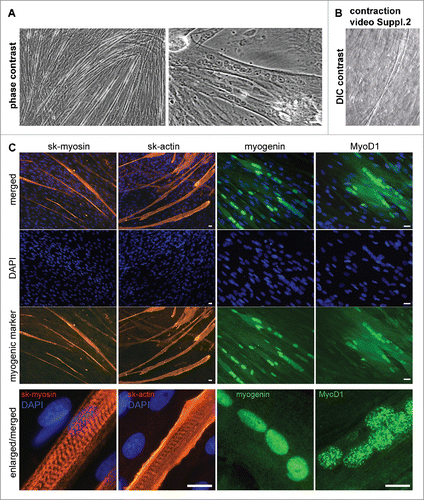
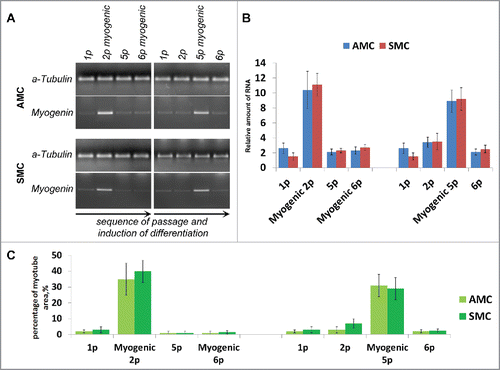
Figure 6. Comparison of AGC, AMC and SMC primary myogenic potential. (A) Phase contrast microcopy and simultaneous skeletal actin and myosin fluorescent staining of primary and induced in myogenic direction AGC, AMC and SMC cultures. One from each typical AGC, AMC and SMC cultures immunofluorescence staining are presented (Bars 100 μm). (B) Analysis of myotube/sk-actinandmyosin-positive areas of primary and induced in myogenic direction AGC, AMC and SMC cultures. Average data for ten AGC and AMC cultures obtained from the same donors and six SMC cultures obtained from other donors are presented (Mean ± SD). (C) The result of one of the representative End-point PCR experiments of MyoD1 and myogenin myogenic factors of one from each AGC, AMC and SMC cultures. α-tubulin mRNA was analyzed as loading control. (D) Relative quantities of MyoD1 and myogenin mRNAs estimated by Real-time qPCR. α-tubulin gene was used for data normalization. Average data for ten AGC and AMC cultures obtained from the same donors and six SMC cultures obtained from other donors are presented (Mean ± SD).
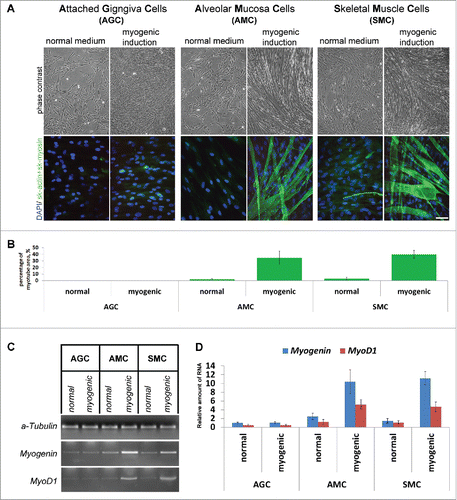
Figure 7. Comparison of myogenic differentiation potential of AMC and SMC at different passages, failure of repeated myogenic differentiation of both cell types. (A) The result of one of the representative End-point PCR experiments of myogenin expression, α-tubulin mRNA was analyzed as loading control. Typical PCR results for one from each AMC and SMC cultures are presented. (B) Relative quantities of myogenin mRNAs estimated by Real-time qPCR. α-tubulin gene was used for data normalization. Average data for ten AMC cultures and six SMC cultures are presented (Mean ± SD). (C) Analysis of myotube/sk-actinandmyosin-positive areas of primary and induced in myogenic direction AMC and SMC at different passages. Average data for ten AMC cultures and six SMC cultures are presented (Mean ± SD).