Figures & data
Figure 1 Study design diagram. BID = twice-daily; FORM = formoterol fumarate; QD = once-daily; TIO = tiotropium bromide; Wk = week.
Figure 2 Timing of spirometry measurements. Spirometry was performed to measure FEV1 and FVC before and after administration of the first dose of study treatment (A) and before and after doses administered during visits at study weeks 4, 8, and 12 (B). FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
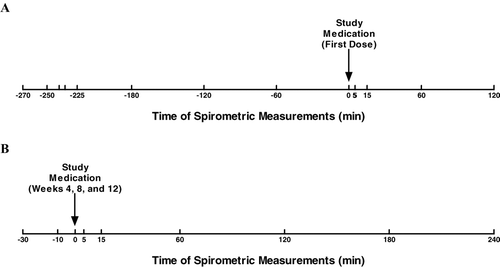
Table 1 Baseline demographic and disease characteristics (randomized patients)
Figure 3 Patient disposition. FORM = formoterol fumarate 12 μ g BID; TIO = tiotropium bromide 18 μ g QD.
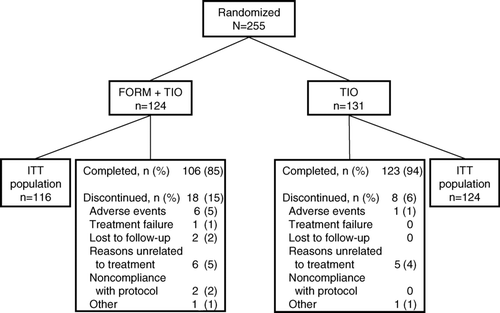
Figure 4 Improvements by treatment visit in normalized forced expiratory volume in 1 second (FEV1) area under the response-time curve from 0–4 hours (AUC0–4h) (A) and normalized forced vital capacity (FVC) AUC0–4h(B). FORM = formoterol fumarate 12 μ g BID; TIO = tiotropium bromide 18 μ g QD. * p < 0.001 compared with TIO.
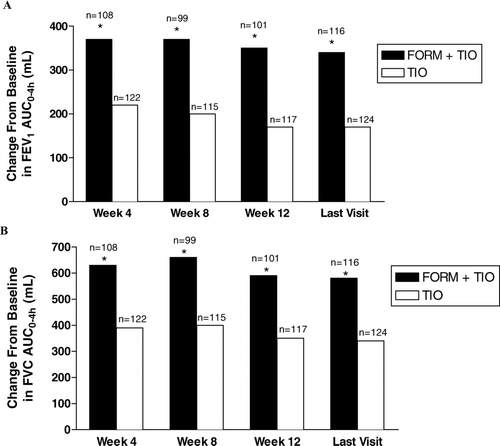
Figure 5 Improvements by treatment visit in trough values for forced expiratory volume in 1 second (FEV1); shown are the averages of values obtained 30 and 10 minutes predose. FORM = formoterol fumarate 12 μ g BID; TIO = tiotropium bromide 18 μ g QD. *p < 0.01; †p < 0.001 compared with TIO.
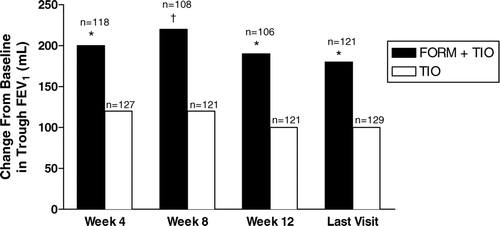
Figure 6 Onset of improvements in postbronchodilator forced expiratory volume in 1 second (FEV1) following administration of the first dose of each study treatment; the FEV1 measurement at t = 0 was obtained immediately before administration of FORM + TIO (n = 118) and TIO (n = 125). FORM = formoterol fumarate 12 μ g BID; TIO = tiotropium bromide 18 μ g QD. *p < 0.001; † p = 0.005 compared with TIO.
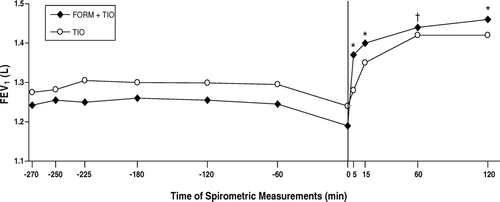
Table 2 Changes from baseline to last visit in patient-centered outcomes
Table 3 Treatment-emergent adverse events reported by ≥ 3% of patients in either group