Figures & data
Figure 1. Impact of treatments on disease progression Citation(9). This figure shows the hypothetical effects of symptomatic and disease-modifying treatments on disease progression in early-start and delayed-start trial models. Disease-modifying treatments provide a sustained alteration in disease progression. Reprinted from COPD: Journal of Chronic Obstructive Pulmonary Disease. Halpin and Tashkin. Defining Disease Modification in Chronic Obstructive Pulmonary Disease. COPD 2009: 6: 211–213 with permission of the publisher Taylor & Francis Ltd (www.tandfonline.com).
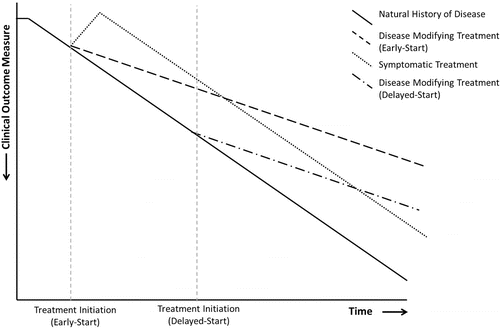
Table 1. Summary of outcome measures used to monitor COPD and emphysema progression (Citation9, 39, 41, 43, 44, 69).
Figure 2. RAPID and RAPID Extension trial design. This figure shows the design of the RAPID trial; subjects in RAPID received A1-PI or placebo for 2 years. During the RAPID Extension trial all non-US subjects received A1-PI for a further 2 years.
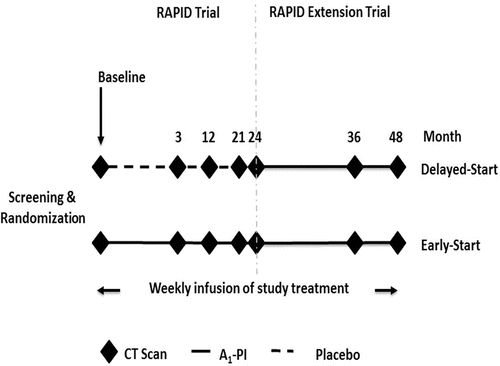
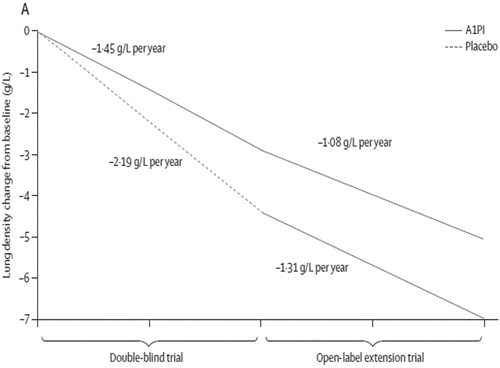
Figure 3. Disease modification in the RAPID and RAPID Extension trials Citation(10). Graph showing the annualised rate of decline in physiologically adjusted P15 (g/L) at TLC over 48 months (ITT population). Reprinted from The Lancet, Vol 386. Chapman, KR et al., Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial, pp. 360–368. Copyright (2015), with permission from Elsevier.