Figures & data
Figure 1. PRISMA flow diagram for the identification of studies included in the meta-analysis concerning the impact of mucolytic agents on COPD exacerbations (A). Diagram displaying the network of the five arms involved in the Bayesian analysis. The links between nodes indicate the direct comparisons between pairs of treatments. The numbers shown along the link lines indicate the number of COPD patients comparing pairs of treatments head-to-head (B).
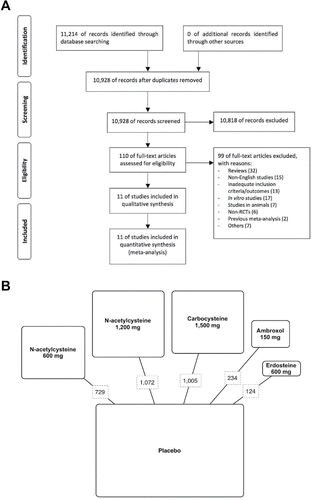
Table 1. Patient demographics, baseline and study characteristics.
Table 2. Summary of the risk of bias for each included study assessed via the Cochrane Collaboration's tool Citation(18).
Table 3. Definition of COPD exacerbations as reported by studies included in the meta-analysis.
Figure 2. Forest plot of pair-wise meta-analysis of the impact of the mucolytic drugs on the odds of COPD exacerbations vs. placebo (A), subset analysis performed on specific mucolytic drugs and doses investigated in high-quality studies (B). Ranking plot for the network of mucolytic agents on the odds of COPD exacerbations (C) in which the treatments have been ranked according to SUCRA (rank of 6 being the worst treatment). Ambr, ambroxol; carbo, carbocysteine; erdo, erdosteine; PCB, placebo; NAC-high, N-acetylcysteine high-dose; NAC-low, N-acetylcysteine low-dose; SUCRA, surface under the cumulative ranking curve. NS, not significant (p ≥ 0.05); * p < 0.05.
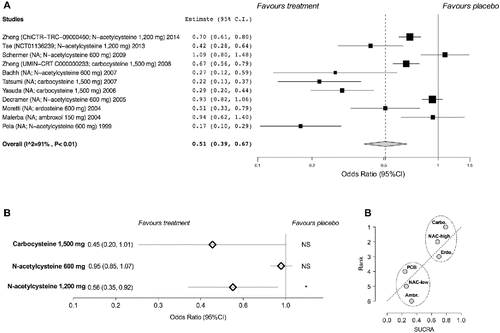
Table 4. SUCRA values for the impact of mucolytic agents in reducing the odds of COPD exacerbations.
Figure 3. Publication bias assessment via funnel plots (left panels) and Egger's test (right panels) for the impact of mucolytic agents (A and B) on the rate of COPD exacerbations vs. placebo, and subset analysis of carbocysteine (C and D) and N-acetylcysteine (E and F). SND, standard normal deviate. *p < 0.1.
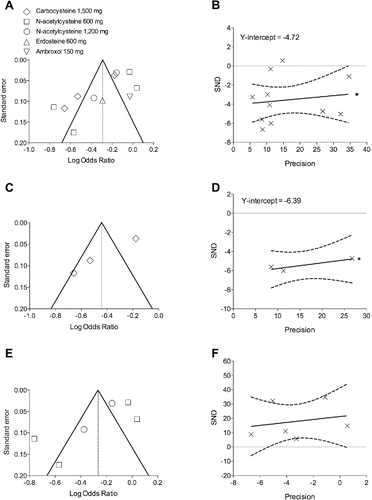
Figure 4. Meta-regression analysis for study and publication characteristics (A–C), disease characteristics (D–F), ethnicity (G) and corticosteroid therapy (H and I). The lower the log odds ratio the greater the treatment effect on reduction of COPD exacerbations. FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; CS, corticosteroids. *p < 0.05: significant interaction between the treatment with mucolytic drugs vs. exacerbation rate and the potential modifier covariates.
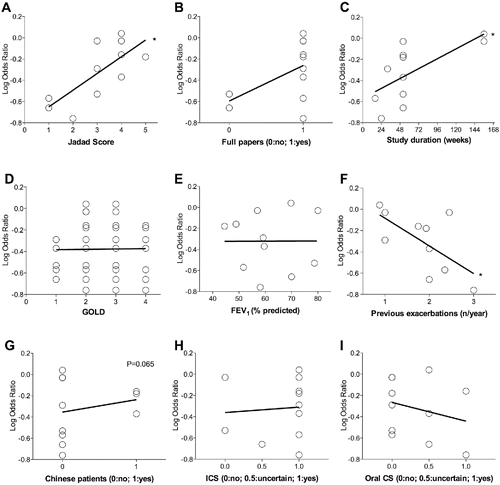
Table 5. GRADE evidence profile: mucolytic agents and COPD exacerbations.
