Figures & data
Figure 1. PRISMA flow diagram for the identification of the full text articles included in the meta-analysis concerning the impact of T/O 5/5 μg FDC vs. monocomponents on the risk of arrhythmia, heart failure, myocardial infarction, and stroke in COPD patients enrolled in RCTs. COPD: chronic obstructive pulmonary disease; CV: cardiovascular; FDC: fixed-dose combination; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs: randomized controlled trials; SAEs: serious adverse events; T/O: tiotropium/olodaterol.
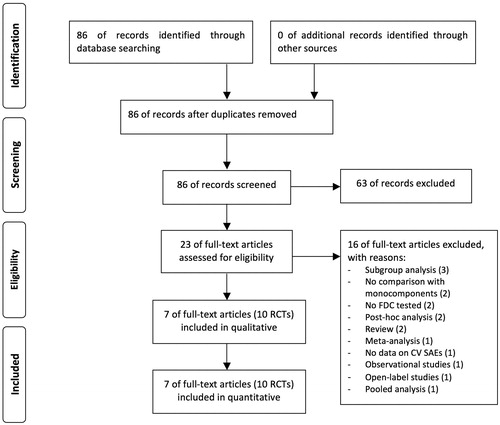
Table 1. Patient demographics, baseline and study characteristics.
Figure 2. Forest plot of pairwise meta-analysis of the impact of T/O 5/5 μg FDC vs. monocomponents on the risk of arrhythmia (A), heart failure (B), myocardial infarction (C), and stroke (D) in COPD patients. Each forest plot reports also the subset analysis with regard to the effect of the class of monocomponents included in the FDC (T/O 5/5 μg FDC vs. T 5 μg or vs. O 5 μg). COPD: chronic obstructive pulmonary disease; FDC: fixed-dose combination; LABA: long-acting β2 adrenoceptor agonist; LAMA: long-acting muscarinic antagonist; O: olodaterol; T: tiotropium.
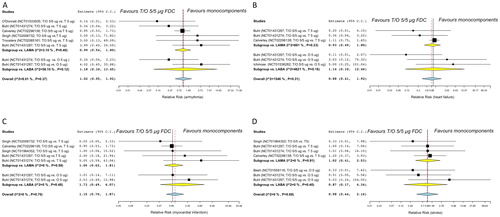
Table 2. Pooled analysis of the frequency of specific CV SAEs (arrhythmia, heart failure, myocardial infarction, and stroke) in COPD patients treated with T/O 5/5 μg FDC and their monocomponents. Data were extracted from RCTs in the repository database ClinicalTrials.gov that reported at least one specific CV SAEs in the investigated arms. The frequencies were ranked in agreement with EMA guideline.
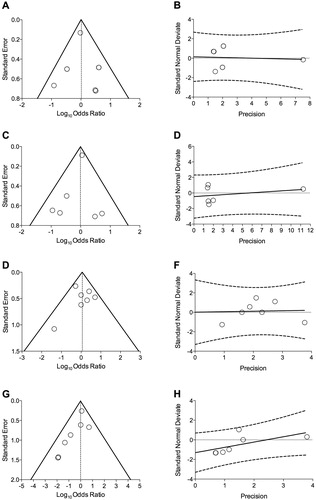
Figure 3. Publication bias assessment via funnel plots (left panels) and Egger’s test (right panels) for the impact of T/O 5/5 μg FDC vs. monocomponents on the risk of arrhythmia (A and B), heart failure (C and D), myocardial infarction (E and F), and stroke (G and H) in COPD patients. COPD: chronic obstructive pulmonary disease; FDC: fixed-dose combination; O: olodaterol; T: tiotropium.