Figures & data
Figure 1 Characterization of the mineral oil overlay model in NRK cells. Ischemia was induced in NRK cells by layering cells with PBS supplemented with 1.5-mM CaCl2 and 2-mM MgCl2 followed by mineral oil. After 3–6 hr, reperfusion was simulated by the removal of the mineral oil and the addition of normal growth medium to the cultures. (a) The impact of ischemia/reperfusion on cell number and cell viability was assessed using the Janus green, neutral red and ATP assays, respectively. Results are expressed as percentage of control from twelve replicate cell culture dishes; * indicates a significant difference in ATP levels as compared to control. (b) Nuclear lysates of ischemic NRK cells were prepared at the indicated times and separated by SDS-PAGE (12%). Following transfer, the blots were probed with a monoclonal anti–HIF-1α antibody. Signals were detected by ECL and autoradiography. Similar results were seen in three replicate experiments.
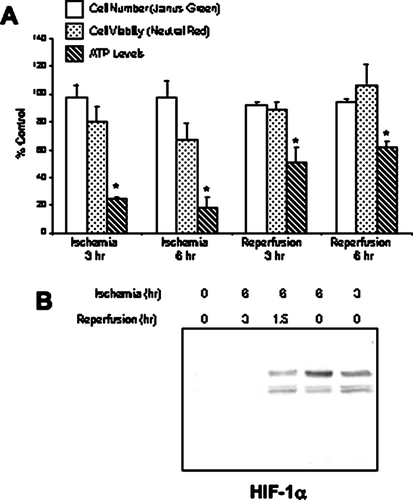
Figure 2 Impact of ischemia on cadherin and catenin protein expression and function in NRK cells. Ischemia was induced in NRK cells by layering cells with PBS supplemented with 1.5-mM CaCl2 and 2-mM MgCl2 followed by mineral oil. (a) NRK cell lysates were prepared at the given times and westerns were performed as previously described. Similar results were seen in three replicate experiments. (b) NRK extracts were immunoprecipitated with a goat polyclonal β-catenin antibody, and the immunoprecipitates were probed with a rabbit polyclonal α -catenin antibody. Following detection, the blots were stripped and reprobed with a monoclonal antibody against β-catenin as a loading control. Similar results were seen in two separate experiments. (c)Morphology of control and ischemic NRK cells, assessed by phase-contrast microscopy. The width of each field is 1600μ m.
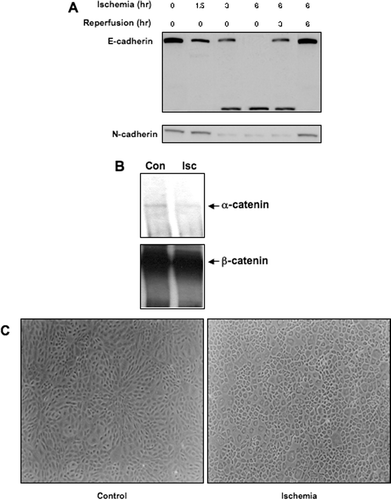
Figure 3 Impact of ischemia/reperfusion on β-catenin signaling in NRK cells. (a) Ischemia was induced in NRK cells by layering cells with PBS supplemented with 1.5-mM CaCl2 and 2-mM MgCl2 followed by mineral oil. NRK cell lysates were prepared at the given times and Westerns were performed as previously described. Similar results were seen in three replicate experiments. The membranes were first probed with the phospho-specific antibodies, stripped and the reprobed with anti-GSK3β or β-catenin antibodies. NRK = control NRK cells incubated in growth medium; PBS = NRK cells incubated with PBS supplemented with Ca+ + and Mg+ + for 6 hr, but not with the mineral oil overlay to induce ischemia.(b) Ischemia was induced in NRK cells by layering cells with PBS supplemented with 1.5-mM CaCl2 and 2-mM MgCl2 followed by mineral oil for 6 hr; cells were reperfused for 3 hr. Cells were fixed in 2% paraformaldehyde, incubated with primary antibody (1:100) overnight at 4°C, with secondary antibody (1:200) 1 hr at room temperature, and detection was via immunofluorescence. Arrows indicate the presence of perinuclear β-catenin signal. The width of each field is 240 μ m; similar results were seen in four replicate experiments. (c) As a positive control, SW480 cells were stained for β-catenin using the same protocol; strong nuclear staining of β-catenin is seen in these cells. The image on the left is β-catenin staining; the image on the right is DAPI nuclear staining. (d) NRK cells transfected with the TOPFlash or FOPFlash reporter constructs were challenged by ischemia/reperfusion and harvested at the indicated time points for luciferase measurement. As a positive control, cells were challenged for 18 hr with 50-mM LiCl2. Results are presented as percentage of control (TOPFlash minus FOPFlash); each data point represents the mean ± SD of 9–12 dishes. (e) Quantiative PCR analysis of cyclin D1, c-myc, and c-jun gene expression in control, ischemic (6 hr) and ischemia–reperfusion (6 hr/3 hr) NRK cells; each data point represents the mean ± SD of 4 replicates.
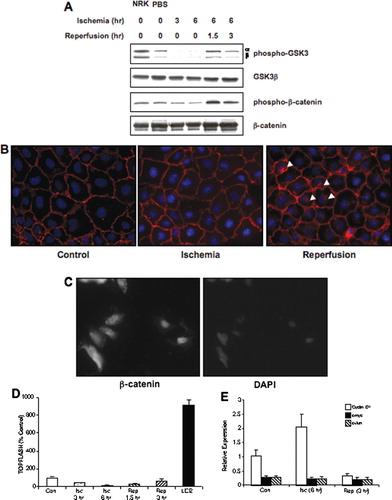
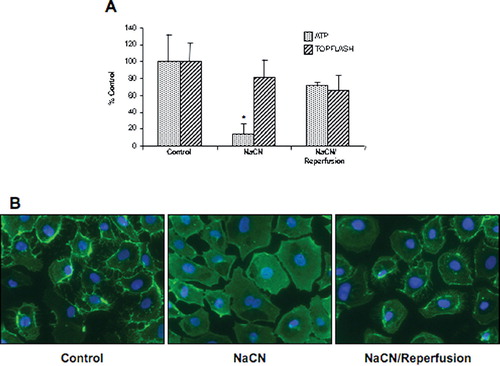
Figure 4 Impact of chemical ischemia/reperfusion on ATP content and β-catenin signaling. (a) NRK cells were challenged with 10-mM NaCN in glucose-free media for 70 min, followed by reperfusion with normal growth media for 1hr. ATP content and β-catenin signaling (ratio of TOPFlash to FOPFlash) were assessed and the results presented as percentage of control for 9–12 dishes. (b) Cells were fixed in 2% paraformaldehyde, a monoclonal anti–β-catenin primary antibody (1:100) incubated overnight at 4°C with secondary antibody (1:200) incubated 1 hr at room temperature, and detection was via immunofluorescence. The width of each field is 240 μ m; similar results were seen in two replicate experiments.