Figures & data
Figure 1 Phosphotyrosine antibodies are specific for Cx43 phosphorylated on Y247 or Y265. Immunoblot analysis of LA-25 cell lysates grown at the nonpermissive (40) or permissive (35) temperature. Replicate immunoblots were probed simultaneously with a monoclonal antibody (NT1) to total Cx43 (upper panel) and antibody against Cx43 phosphorylated on Y247 (Ab pY247) or Y265 (Ab pY265) (lower panel) as indicated. Antibodies were coincubated with immunizing peptides (Pt Y247 or Pt Y265) as indicated to demonstrate the specificity of each phosphospecific antibody. Note that antibody binding in the western blots was detected via fluorescence with the Odyssey imaging system allowing simultaneous detection of the phosphospecific and total Cx43 antibodies resulting in a white on black image for each channel.
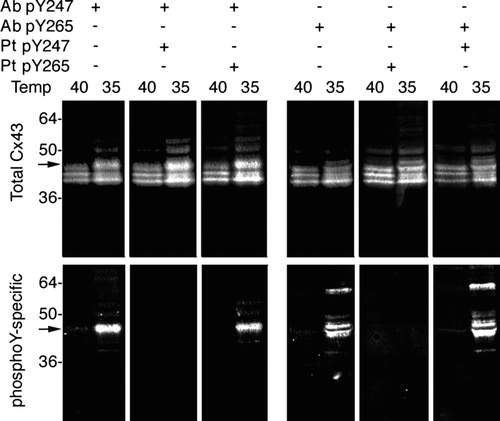
Figure 2 pY247 and pY265 antibodies recognize Cx43 in a subset of gap junction plaques. Immunofluorescence was performed on LA-25 cells at the permissive (35°C) or nonpermissive temperature (40°C). Large panels show a field of cells labeled with antibody to total Cx43 in green and antibodies to phosphotyrosine 247 (pY247) or 265 (pY265) in red, overlay (Ovl) is seen as yellow. Small panels to the right show a magnified view of boxed areas with individual channels separated to highlight the colocalization of the Cx43 in plaques (magnification bar = 25 μ m).
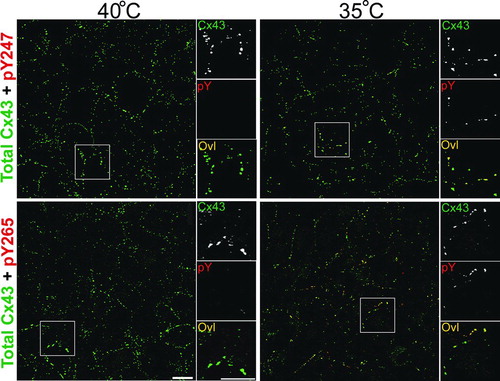
Figure 3 pS279/282 specifically recognizes Cx43 phosphorylated on S279/282 in response to PDGF, EGF, or TPA stimulation. (Upper panel) Immunoblot analysis of 293T cell lysates after 10 min treatment with PDGF, EGF or TPA (all 10 ng/ml). Blots were probed with antibody to total Cx43 (NT1 signal) and pS279/282 (pS279/282 signal). (Lower panel) MDCK cells were transfected with wild-type Cx43 (WT43) or Cx43 containing serine to alanine mutations at S279, S282, or both (S279A, S282A, and S279/282A, respectively). Blots were probed with antibody to total Cx43 (NT1 signal) or pS279/282 (pS279/282 signal). Signals were quantified and ratios of pS279/282 to total Cx43 were calculated (graph, lower panel). Error bars depict standard error and differences between WT43 and all 3 mutants were statistically significant (Student's t test p value < 0.0001, n = 3).
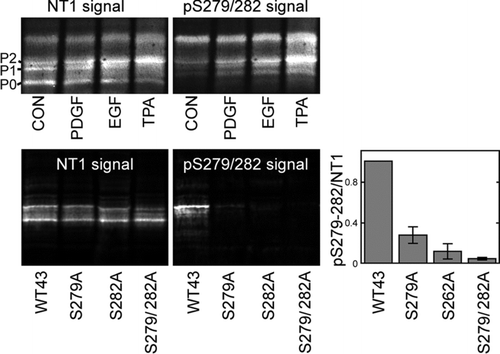
Figure 4 Active v-src leads to increased phosphorylation on specific tyrosine and serine residues in Cx43. Immunoblot analysis of LA-25 cells grown at the permissive (35) and nonpermissive (40) temperature. Replicate blots were probed with antibodies to total Cx43 (NT1) and a series of phosphospecific antibodies. (Upper panels) NT1 and phosphotyrosine antibodies pY247 (A) and pY265 (B), CT1, recognizing unmodified S364/365 (C) and phosphorylated S262 (pS262) (D). (Lower panels) NT1 and antibodies recognizing phosphorylated S279/282 (pS279) (E) and S368 (pS368) (F). Signals were quantified and the ratio of phosphospecific antibodies to total Cx43 were calculated (G). Differences between permissive and non-permissive temperature was significant for both the pY247 and pY265 antibodies (Student's t test p value < 0.001, n = 3).
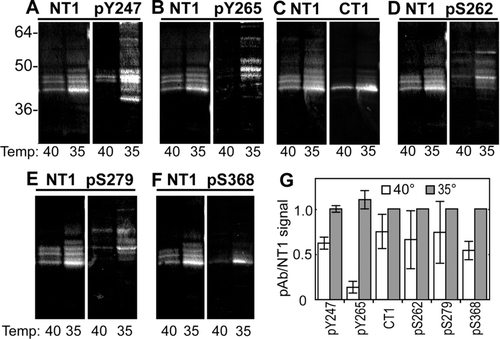
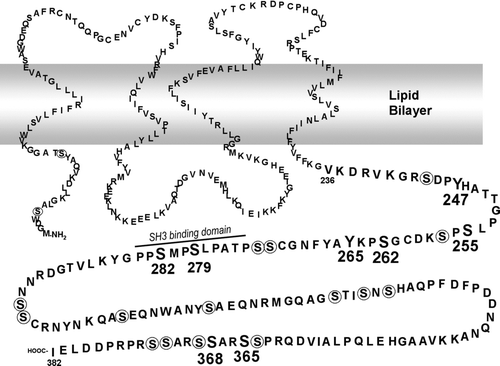
Figure 5 Model of Cx43 with phosphorylated residues and SH3 binding domains indicated (modified from Lampe and Lau Citation2000). Residues to which phosphospecific antibodies have been made are shown in large bold print. Other serine residues are circled. A SH3 binding domain involved in v-src interaction is noted (Kanemitsu et al. Citation1997).