Figures & data
Figure 1 Diagram illustrating the surface protein biotinylation procedure for detection of hemichannels in living cells. Cultured cells are incubated at 4°C with the Sulfo-NHS-SS-Biotin (B), which binds connexins/pannexins forming hemichannels (HC) with respect to those forming intercellular channels at the junctional plaques (IC). After biotin removal, cells are washed with a glycine-containing solution and homogenized in the presence of proteases and phosphatases inhibitors. Total protein contents are measured and corrections are made to ensure that each sample has the same amount of proteins. Then, the same amount of avidin-agarose is added to each sample to pull down the biotin/surface protein complexes. After pull down, preparations are washed and eluted with an acid buffer, to release the surface proteins from the biotin/avidin complexes (BAC). Isolated surface proteins are blotted and probed with antibodies against the connexin/pannexin of interest.
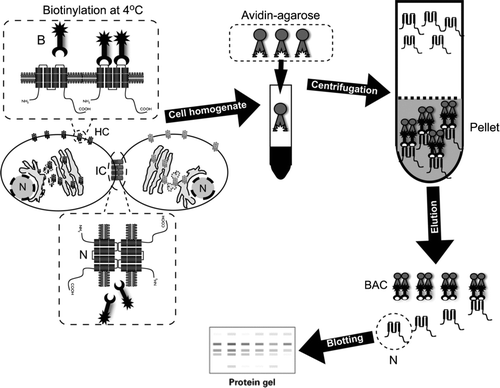
Figure 2 Surface Cx43 corresponds to a small amount of total Cx43 in HeLa transfectants. (Upper panel) Representative immunoblots showing the relative levels of total (left) or biotinylated Cx43 (right) in HeLa cells stably expressing wild type Cx43 (HeLa-Cx43). (Lower panel) Normalized densitometric values; the band intensity detected in the biotinylated surface protein fraction relative to the total protein levels of connexin taken as 100%. Cells were studied under control conditions and in the presence of physiological concentrations of divalent cations (1.8 mM Ca2 + and 1 mM Mg2 +). Determination of surface Cx43 with respect to total protein was performed by blotting different amounts of total (1, 10, 20, 40, 80, and 160 μ g) and a sample of biotinylated proteins in the same gel. Densities were measured, a fitting curve was constructed and the protein amount of the biotinylated sample was determined by interpolation. Each bar represents average ± SEM of three independent experiments; **p < 0.01. NP and P2-P3 denote the unphosphorylated and phosphorylated forms of Cx43, respectively. Statistic analysis was performed using descriptive statistics and Microscoft Excel software.
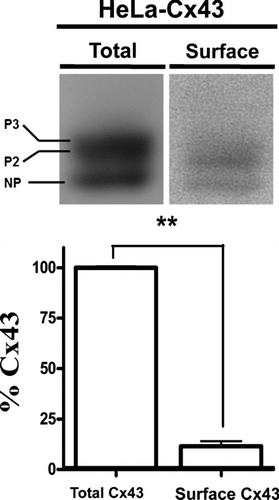
Figure 3 Sulfo-NHS-SS-Biotin neither enters cells through open hemichannels nor blocks the ethidium uptake. (A) Structure of Sulfo-NHS-SS-Biotin. (B) Surface Cx43 levels of mouse cortical astrocytes incubated with Sulfo-NHS-SS-Biotin in the presence of 1.8 mM Ca2 + and 1 mM Mg2 + (control) or in the absence of extracellular divalent cations (Ca2 +/Mg2 +-free). (C) Surface Cx43 levels of HeLa-Cx43 in control conditions (control), after treatment with 2.5 μ M 4-Br-A23187 for 50 min or after treatment with 2.5 μ M 4-Br-A23187 for 50 min and incubated with Sulfo-NHS-SS-Biotin in the presence of 200 μ M La3 + to block connexin hemichannels. NP and P2-P3 denote the unphosphorylated and phosphorylated forms of Cx43, respectively. (D) The Etd (5 μ M) uptake rate of HeLa-Cx43eGFP cells before and after addition of 500 μ g/ml Sulfo-NHS-SS-Biotin. Cells were recorded at room temperature in the presence of 1.8 mM Ca2 + and 1 mM Mg2 +, pH = 7.4. Each bar represents the mean ± SEM. Each experiment included time-lapse measurements of 20 cells and the number of independent experiments is indicated within each bar; ns = not significant (p > 0.05, U test).
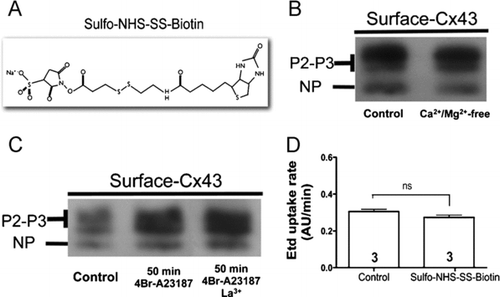
Figure 4 Removal of extracellular divalent cations increases the ethidium uptake in HeLa cells expressing Cx43 and the response is sensitive to a connexin hemichannel blocker. (A) Representative fluorescence photomicrography of the Etd (5 μ M) uptake in parental (HeLa-parental) or HeLa-Cx43 cells after 10 min exposure to a Ca2 +/Mg2 +-free solution (Ca2 +/Mg2 +-free). The Ca2 +/Mg2+-free solution was prepared in sterile-filtered Sigma water with no added divalent cations plus 5 mM EGTA, pH = 7.4. Bar = 20 μ m. (B) Representative time-lapse recording of the Etd (5 μ M) uptake in HeLa-parental or HeLa-Cx43 cells in control condition, bathed for 10 min with a solution lacking divalent cations (Ca2 +/Mg2 +-free) and after addition of 200 μ M La3 +. Cells were recorded at room temperature and pH = 7.4. Each point in the graph represents mean ± SEM of 20 cells.
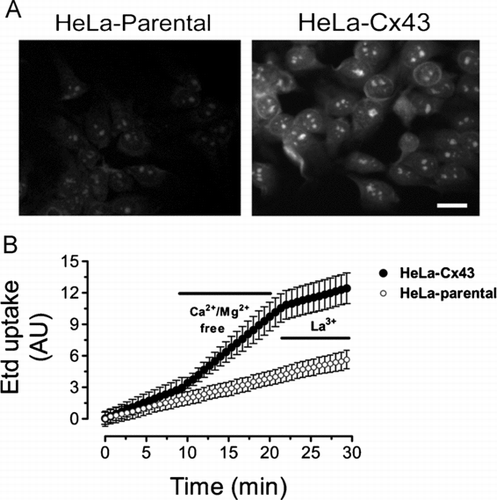
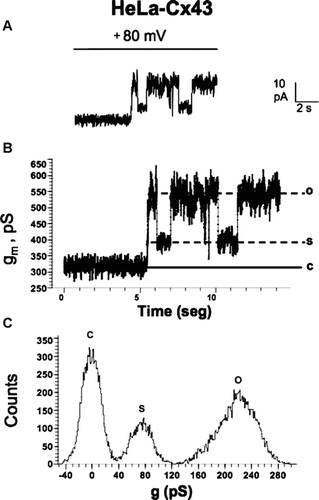
Figure 5 Records of unitary hemichannel currents in HeLa-Cx43 cells. Whole-cell voltage clamp recording in HeLa-Cx43 cells in the presence of extracellular divalent cations. The ionic composition of the extracellular and pipette solution was as described by Contreras et al. 2003. (A) A step from 0 to +80 mV elicited discrete current events in control cells. (B) Conductance values of record shown in A. The conductance value between the close state (c) and substate (s) or fully open state (o) correspond to ∼ 75 pS and ∼ 220 pS, respectively. (C) Frequency (counts) distribution of conductance values shown in B.