Figures & data
Figure 1 N2A and MIN6 cells differ in connexin but not pannexin expression. (A) RT-PCR showed that N2A cells express pannexin1 (Px1) and pannexin2 (Px2), as well as the P2X7 receptor (P2X7r), but not pannexin3 (Px3) and either connexin36 (Cx36) or connexin43 (Cx43). In contrast, WT MIN6 cells express Px1, P2X7r, and Cx36, little Px2, but no Px3 and Cx43. (B) Western blot of whole extracts and purified membranes showed that both wild type (WT) and Cx36-null MIN6 cells (AS) expressed pannexin1 (Panx1), and inserted the protein into the cell membrane. Both cell types also expressed the P2X7 receptor, which, however, could not be detected on purified membrane extracts of MIN6 cells. (C) Immunofluorescence confirmed the presence of pannexin1 in both the cytoplasm and the membrane of N2A (right panel) and MIN6 cells (left panel). Bar, 20 μm.
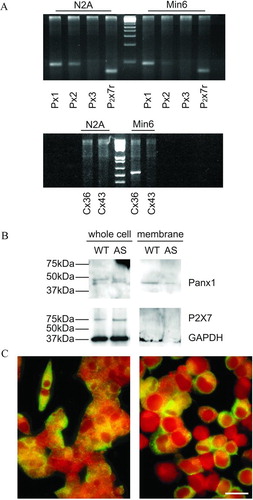
Figure 2 N2A cells express functional “hemichannels.” Time course of ethidium bromide uptake by N2A cells (A) stimulated with 300 μM BzATP (n = 34 cells, 100% of the cells showed small uptake in a Ca2 +-containing medium; (B) exposed to a Ca2 +-free KRB (n = 34 cells, 81% of the cells showed uptake [solid symbols], but 19% did not [open symbols]); or (C) mechanically stressed by exposure to a 50% diluted KRB (n = 35 cells, 63% of the cells showed uptake [solid symbols], but 37% did not [open symbols]). Data are mean ± SEM of three experiments.
![Figure 2 N2A cells express functional “hemichannels.” Time course of ethidium bromide uptake by N2A cells (A) stimulated with 300 μM BzATP (n = 34 cells, 100% of the cells showed small uptake in a Ca2 +-containing medium; (B) exposed to a Ca2 +-free KRB (n = 34 cells, 81% of the cells showed uptake [solid symbols], but 19% did not [open symbols]); or (C) mechanically stressed by exposure to a 50% diluted KRB (n = 35 cells, 63% of the cells showed uptake [solid symbols], but 37% did not [open symbols]). Data are mean ± SEM of three experiments.](/cms/asset/50069b5d-0170-4e09-8deb-2e7af050d918/icac_a_301591_uf0002_b.gif)
Figure 3 MIN6 cells do not express functional “hemichannels.” Time course of ethidium bromide uptake recorded under the same conditions used to screen N2A cells failed to reveal any uptake of ethidium bromide by MIN6 cells (A) stimulated with 300 μ M BzATP (n = 20 cells); (B) exposed to a Ca2 +-free KRB (n = 20 cells); or (C) mechanically stressed by a 50% hypotonic shock (n = 35 cells). Data are mean ± SEM of three experiments.
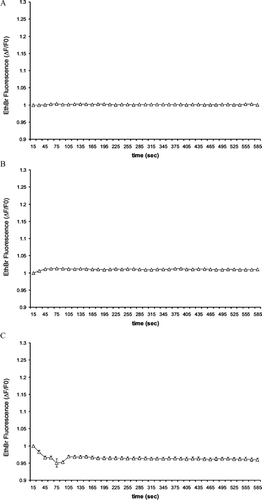
Figure 4 MIN6 cells release ATP only via the exocytotic route. Stimulation of wild-type MIN6 cells (MIN6) by glucose elicited an ATP release (solid column), which was increased by TEA (grey column) and even further in the absence of extracellular Ca2 + (hatched column), but was prevented by the exocytosis blocker diazoxide (open column). Similar observations were made in a clone of MIN6 cells that lacks Cx36 gap junctions (MIN6-AS), as a result of transfection with an antisense Cx36 cDNA.
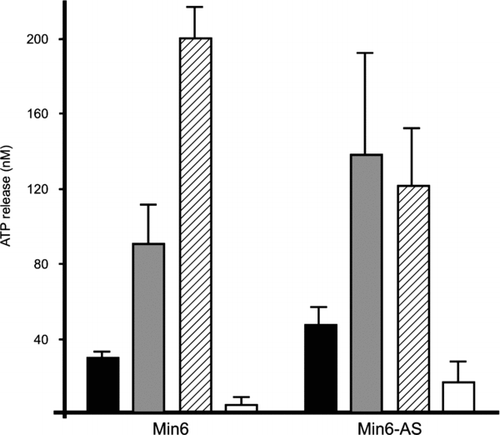
Figure 5 N2A but not MIN6 cells show “hemichannels” after supra-physiological depolarization. (A) Bar histogram of the mean total membrane conductance measured at +100 mV during a voltage ramp from −60 to +100mV. Single N2A and wild-type MIN6 cells were patch-clamped and exposed to a ramp of increasing depolarization steps. For depolarizations above +30 mV, both N2A and MIN6 cell types showed outward currents, with total membrane conductance of about 0.7 nS in MIN6 cells and 1.5 nS in N2A cells. Currents recorded from N2A but not from MIN6 cells were reduced by carbenoxolone (see text), indicating the existence in N2A cells of functional “hemichannels” presumably made by pannexin1. (B) Frequency distribution of the number of MIN6 (black bars) and N2A (gray bars) cells displaying outward currents measured at +100 mV.
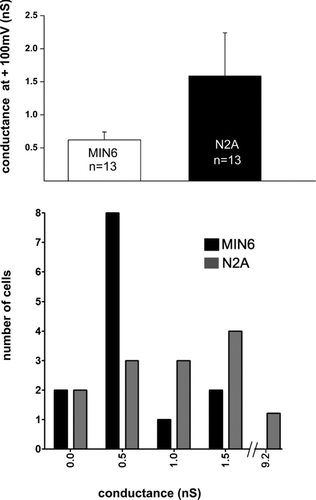
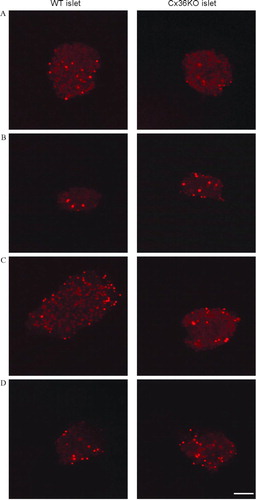
Figure 6 Primary islet cells do not express functional “hemichannels.” Confocal microscopy views of pancreatic islets isolated from control (left column) and homozygous Cx36-null transgenic mice (right column), after a 10-min exposure to control KRB (A); stimulation by 300 μ M BzATP (B); exposure to Ca2 +-free KRB (C); or mechanical stress by a 50% hypotonic medium (D). In both types of islets, none of the experimental conditions significantly increased the number of ethidium bromide-stained cells, over the basal number observed under control conditions (A). In all cases, the tracer labeled only the cell nuclei of presumably damaged cells. Bar, 65 μ m.