Figures & data
Figure 1 Effect of intermittent and continuous PTH(1-34), and/or β-GA on MLO-A5 cell mineralization. (A) At the third day after cell plating, MLO-A5 cells were treated with 1 × 10− 8M PTH(1-34) either continuously (Cont) for 48 h or intermittently (Inter) for 4 h every 48 h in the presence (+) or absence (−) of β-GA. After 3, 6, 9, and 12 days of culturing, the cells were fixed and mineralization was analyzed by von Kossa staining. (B) The degree of mineralization was quantified by densitometry (ImageJ, NIH). Non-β-GA (-) verse β-GA (+) samples with corresponding treatment regimes: **, ρ < 0.01 and ***, ρ < 0.001. The data are presented as the mean ± SEM and n = 3.
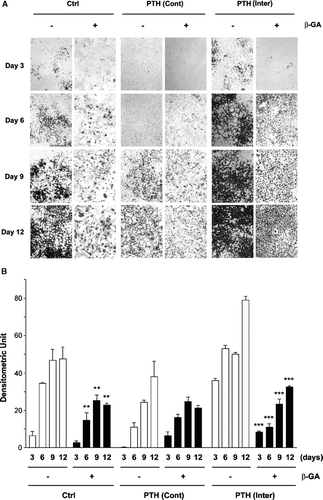
Figure 2 Upregulation of intercellular coupling observed in both intermittent and continuous treatments with PTH(1-34). MLO-A5 cells were treated in the absence or presence of 1 × 10− 8M PTH(1-34) either continuously (Cont) for 48 h or intermittently (Inter) for 4 h for a total of 48 h or were incubated with gap junction inhibitor β-GA. Intercellular coupling between cells was analyzed using the scrape-loading dye transfer assay. Lucifer yellow (LY) was used as a fluorescent tracing molecule for gap junctional coupling and rhodamine dextran (RD) serves as a marker for dye-loaded cells. The percentage of dye transfer was referred to as the percentage of RD-receiving cells that form gap junctions. The degree of dye transfer in intermittent (Inter) or continuous (Cont) PTH treated versus non-treated (Ctrl) or β-GA treated cells, ***ρ < 0.001. The data are presented as mean ± SEM and n = 3.
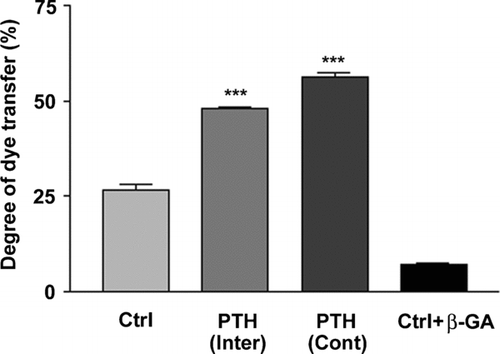
Figure 3 No effect of PTH(1-34) on hemichannel dye uptake. (A) MLO-A5 cells cultured at low cell density without cell-cell contact were treated in the absence (Ctrl) or presence of 1 × 10− 8 M PTH(1-34) (PTH) or PTH(1-34) plus 5 mM EGTA (PTH + EGTA) for 30 min and dye uptake was conducted with LY/RD. For dropping (DP) analysis, LY/RD dyes were directly delivered to the cell by pipetting. bar: 100 μ m. (B) The numbers of MLO-A5 cells with dye uptake in the absence or presence of PTH(1-34), PTH(1-34) plus EGTA or dropping were counted and quantified. Ctrl, PTH or PTH+EGTA versus DP: **ρ < 0.01. The data are presented as mean ± SEM and n = 3.
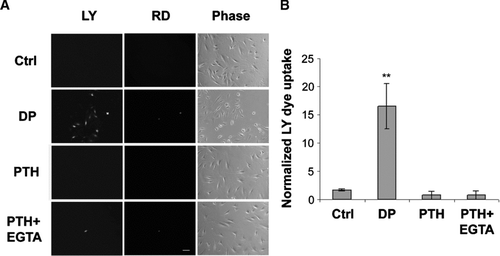
Figure 4 Cx43 expression and intracellular distribution in MLO-A5 cells in response to intermittent and continuous treatment with PTH(1-34). MLO-A5 cells were treated with 1 × 10− 8M PTH(1-34) either continuously (Cont) for 48 h or intermittently (Inter) for 4 h per 48 h culture or not treated for 48 h (Ctrl). (A) The above treated cells were lysed, and crude membranes were isolated and immunoblotted with affinity-purified anti-Cx43 or anti-β-actin antibody. The protein band intensity was measured by densitometry and the intensity ratio of Cx43 versus β-actin was calculated (right panel). (B) The above treated cells were immunolabeled with affinity purified anti-Cx43 antibody followed by FITC-conjugated goat anti-rabbit secondary antibody. Upper panels, low magnification; bar: 100 μ m. Lower panels, high magnification; bar: 10 μ m. (C) Cells were treated in the absence (Ctrl, lane 1) or presence of PTH(1-34) intermittently (lane 3) or continuously (lane 4) or with 1 μ M thapsigargin (The) (lane 2). The cell lysates were immunoblotted with anti-CHOP antibody.
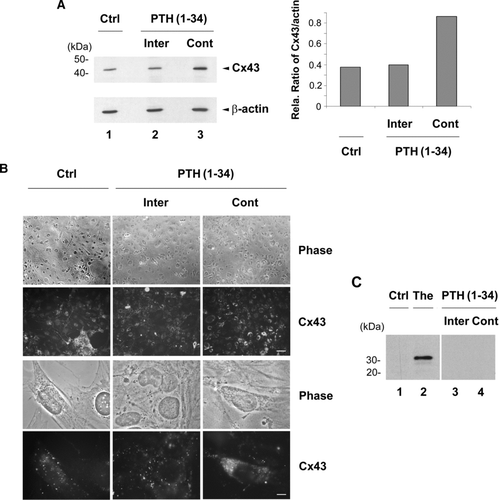
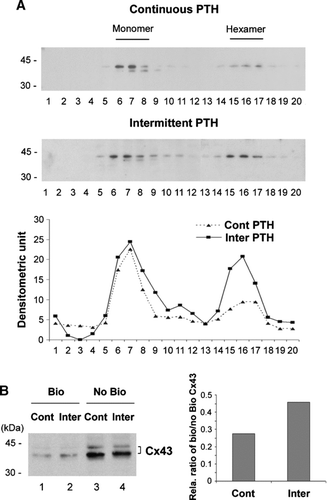
Figure 5 Intermittent PTH promoted connexon formation and surface expression of Cx43. (A) Sucrose gradient analysis of Cx43 connexons isolated from PTH(1-34) treated cells. MLO-A5 cells were treated with PTH(1-34) either continuously or intermittently for 48 h. The crude membranes were solubilized by 1% Triton-X-100 and separated by centrifugation, and supernatants were fractionated on a linear gradient of 5–20% sucrose. The collected fractions were immunoblotted with affinity purified anti-Cx43 antibody. The protein band intensity from sucrose gradient fractions of continuous and intermittent PTH treated samples was measured by densitometry (lower panel) (n = 3). (B) After the treatment with continuous (lanes 1 and 3) or intermittent (Inter) (lanes 2 and 4) PTH(1-34), cells were treated with biotin. The cells were lysed and equal amounts of total protein were applied to avidin-conjugated beads to isolate biotin-labeled proteins. The biotinylated (Bio) Cx43 that bound to avidin beads (lanes 1 and 2) and non-biotinylated (No Bio) Cx43 that flew through the beads (lanes 3 and 4) were detected by Western blots by using anti-Cx43 antibody. The protein band intensity was measured by densitometry and the intensity ratio of biotinylated (Bio) verse non-biotinylated (No Bio) Cx43 was calculated (right panel).