Figures & data
Table 1. Contact angles of water on glass with different modifications.
Figure 1. PE deficiency impairs E. coli adhesion onto cells. (A) Representative confocal images showing the adhesion of four fluorescently-stained (SYBR Green II) E. coli strains on human THP-1-derived macrophages. Upper panel (from left to right): DH5α, PE + PC− strain, PE−PC+ strain, and PE−PC− strain, respectively; bottom panel: enlarged images (the fluorescently stained bacteria are clearly evident) from the boxed areas in upper panel. (B) Representative confocal images showing the adhesion of the fluorescently-stained (SYBR Green II) E. coli strains on mouse RAW264.7 macrophages. (C, D) Statistical analysis of the bacteria adhered on THP-1-derived and RAW264.7 macrophages, respectively (the number of bacteria per cell. ***, p < 0.001 compared with DH5α/PE + PC−).
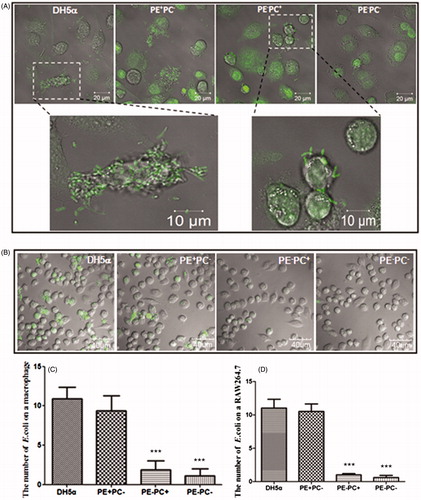
Figure 2. PE deficiency impairs E. coli adhesion onto substrates which is not related to hydrophobicity. (A) Representative images showing the adhesion of the four bacterial types on different substrates (panels from top to bottom: glass, hydrophilic glass, and OTS-glass, respectively). (B) Statistical analysis of the bacteria adhered on the substrates (the number of bacteria per field as a representative image showed in A. *, p < 0.05; ***, p < 0.001 compared with the glass groups; #, p < 0.05 compared with PE+ strains).
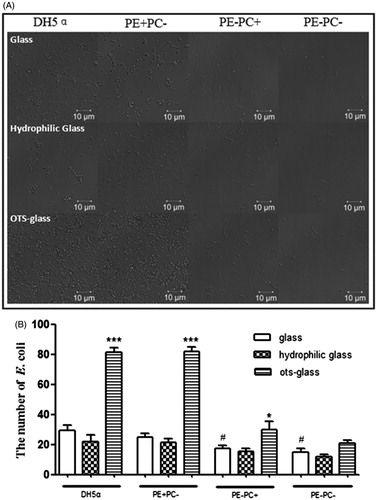
Figure 3. LPS depletion by EDTA significantly impairs the adhesion of the four E. coli strains onto glass coverslips. *, p < 0.05; **, p < 0.01 compared with the corresponding controls; #, p < 0.05 compared with PE + strains (i.e. DH5α and PE + PC− groups).
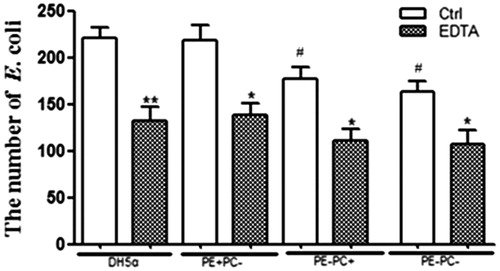
Figure 4. The antibody blocking of cell-surface CD14 (a putative co-receptor for LPS recognition) inhibits E. coli adhesion onto cells. (A) Left two panels: CD14 was fluorescently stained on fixed THP-1 cells and THP-1-derived macrophages, respectively; right two panels: the adhesion of fluorescently-stained (SYBR Green II) PE+ E. coli strains on THP-1-derived macrophages pre-treated with anti-CD14 mAb. (B) Left panel: CD14 was fluorescently stained on fixed RAW264.7 cells; right two panels: the adhesion of fluorescently-stained (SYBR Green II) PE+ E. coli strains on RAW264.7 cells pre-treated with anti-CD14 mAb. (C) Statistical analysis of the bacteria adhered on macrophages pre-treated with or without anti-CD14 mAb (the number of bacteria per cell. ***, p < 0.001 compared with the corresponding controls).
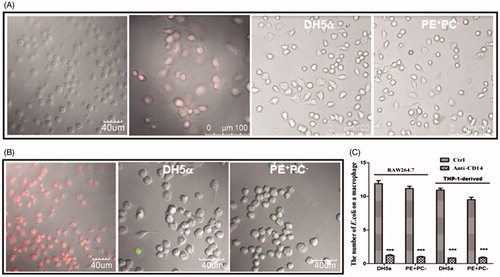
Figure 5. PE deficiency downregulates LPS biosynthesis of E. coli. (A) Silver-stained SDS-PAGE pattern of LPS from the four E. coli strains. (B) Silver-stained SDS-PAGE pattern of LPS from the bacteria treated with EDTA or (C) from the supernatants. Lanes 1 and 6: standard LPS; lanes 2–5: DH5α, PE+PC−, PE−PC+, and PE−PC− strains, respectively. The origin of electrophoretic mobility is on the top.
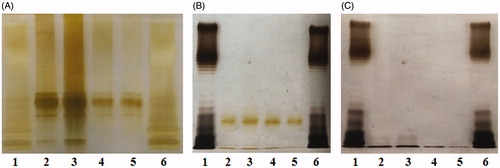
Figure 6. High galactose/lactose cultivation enhances LPS synthesis of PE-deficient E. coli strains. PE−PC− (A) and PE−PC+ (B) bacteria were cultivated in the corresponding medium but supplemented with high sugar (glucose, galactose, glucose-galactose mixture, and lactose, respectively) at different concentrations (0.5%, 1%, and 2%, respectively). Showed are the silver-stained SDS-PAGE patterns of LPS from these bacteria. The first and last lanes are standard LPS; the second lanes are PE- bacteria without high sugar cultivation.
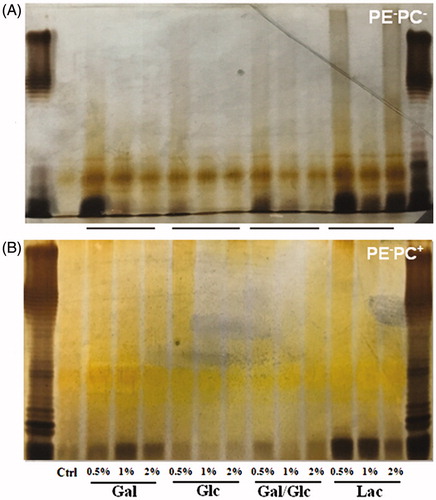
Figure 7. High galactose/lactose cultivation promotes the adhesion of PE-deficient E. coli strains onto substrates. (A) Representative images showing the substrate adhesion of PE−PC+ (upper panel) and PE−PC− (lower panel) bacteria cultivated with high sugar (panels from left to right: no sugar, 1% galactose, 1% glucose, 1% galactose-glucose mixture (1:1), and 1% lactose, respectively). The bacteria were fluorescently stained with SYBR Green II. (B) Statistical analysis of the bacteria adhered on substrates (the number of bacteria per field as a representative image showed in A. **, p < 0.01; ***, p < 0.001 compared with the corresponding controls).
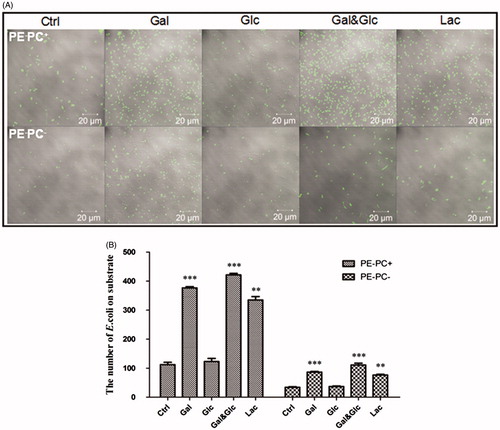
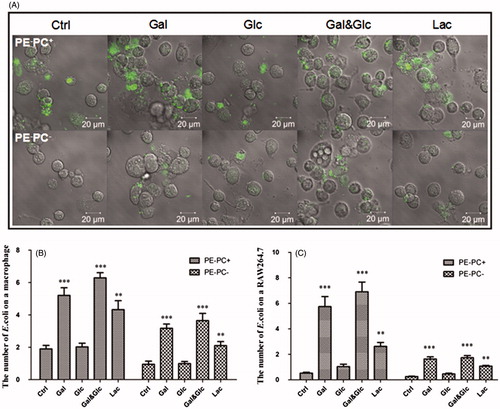
Figure 8. High galactose/lactose cultivation increases the adhesion of PE-deficient E. coli strains onto macrophages. (A) Representative images showing the adhesion of PE−PC+ (upper panel) and PE−PC− (lower panel) bacteria cultivated with high sugar onto macrophages. The bacteria were fluorescently stained with SYBR Green II. (B) Statistical analysis of the PE-deficient bacteria adhered on THP-1-derived macrophages (the number of bacteria per cell. **, p < 0.01; ***, p < 0.001 compared with the corresponding controls). (C) Statistical analysis of the PE-deficient bacteria adhered on RAW264.7 macrophages (**, p < 0.01; ***, p < 0.001 compared with the corresponding controls).