Figures & data
FIG. 2 Lung Cr burdens at Day 0 (i.e., pre-infection) and Day 3 of infection with Listeria. Each bar represents the average burden ([A] ng Cr; [B] ng Cr/g lung) (± SE) in the lungs of 5 (Day 0; solid bar) or 10 (Day 3; hatched bar) rats/treatment regimen. In Day 0 sets; ‡value significantly (p < 0.05) different from that in Cr(II) and soluble Cr(VI) rats. In Day 3 sets, *value significantly (p < 0.05) different from that in rats in all other groups; #value significantly (p < 0.05) different from that in Cr(II) and Cr(III) rats.
![FIG. 2 Lung Cr burdens at Day 0 (i.e., pre-infection) and Day 3 of infection with Listeria. Each bar represents the average burden ([A] ng Cr; [B] ng Cr/g lung) (± SE) in the lungs of 5 (Day 0; solid bar) or 10 (Day 3; hatched bar) rats/treatment regimen. In Day 0 sets; ‡value significantly (p < 0.05) different from that in Cr(II) and soluble Cr(VI) rats. In Day 3 sets, *value significantly (p < 0.05) different from that in rats in all other groups; #value significantly (p < 0.05) different from that in Cr(II) and Cr(III) rats.](/cms/asset/e8eded58-70be-4ffd-bfea-3fc9552e3926/iimt_a_171811_uf0002_b.gif)
FIG. 3 Average retention of Cr in the lungs of Listeria-infected rats in each Cr treatment group. Each bar represents the average retention (%; ± SE) of Day 0 burden in the lungs of 10 Day 3 rats per treatment regimen. Data analyzed in terms of ng Cr (solid bar) or of ng Cr/g lung (hatched bar). *Value significantly (p < 0.05) different from that in rats in all other groups; ‡value significantly different from that in rats in the Cr(III) group.
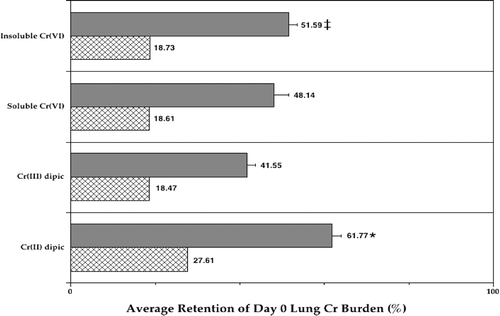
FIG. 4 Listeric (bacterial) burdens in the lung of rats at each day post-infection. (A) CaCrO4; (B) Na2CrO4; (C) Cr(III)dipic; (D) Cr(II)dipic regimen. Values shown with ♦ represent the mean (±SE) of 6, 8 and 10 rats at 24, 48, and 72 hours post-infection/Cr regimen, respectively; values indicated with ○ represent mean (±SE) of 5–6 air control rats at same timepoints. Statistical significance (p value) of result (Cr vs. air)—when present—is indicated.
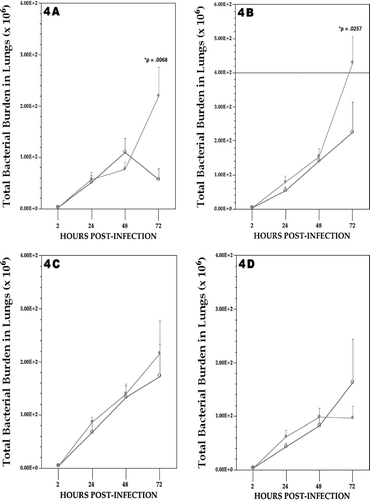
FIG. 5 Relative difference in listeric (LM) burden in the lungs of rats at Day 3 post-infection. Each bar represents the mean (± SE) average percentage differences in Listeria levels (compared to those in air controls) in the lungs of 10 Day 3 rats/indicated treatment regimen. *Value significantly (p < 0.05) different from that in rats in 100 μg Cr/m3 insoluble CaCrO4 group. ‡Value significantly (p < 0.05) different from that in air control rats.
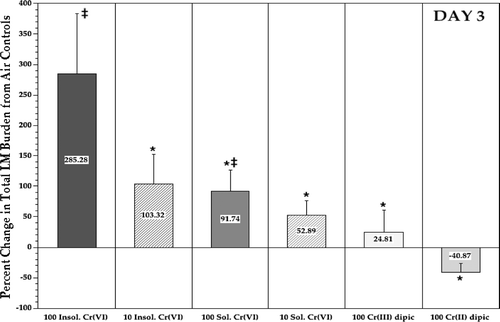
FIG. 6 Relative difference in listeric burden in the lungs of rats at Day 3 post-infection as a function of Day 0 lung Cr burdens. Each bar represents the mean (n = 10, Day 3 rats/indicated treatment regimen; ±SE) average percentage differences in Listeria levels (LM; solid bar) or of total Listeria/g lung (LM/g; hatched bar) compared to respective values in air controls, in the context of ng Cr in lungs at Day 0. ‡Value significantly (p < 0.05) different from that in rats in insoluble CaCrO4 group; #value significantly (p < 0.05) different from that in Na2CrO4 rats.
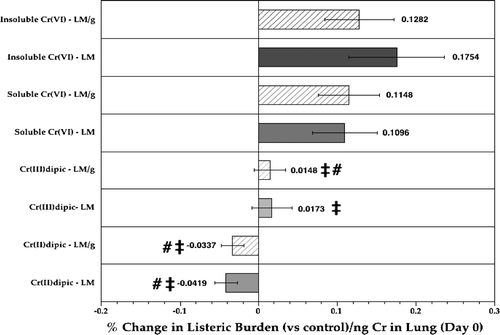
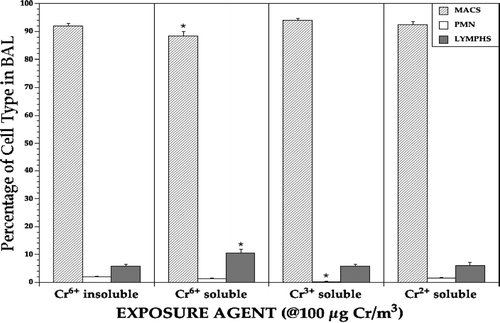
FIG. 7 Immune cell population distribution in the lavages of lungs of rats at Day 0 pre-infection. Each bar represents the mean (±SE) average percentage of the indicated cell type among all cells recovered from the lungs of 5 Day, 0 rats/indicated treatment regimen. *Value significantly (p < 0.05) different from that in rats in the other treatment regimens.