Figures & data
Figure 1. Treatment schedules: mice were treated with 1 (P1), 10 (P10) or 30 (P30) g/kg BW of P. aquilinum and supplemented with B1 vitamin in water ( 10 mg/ml) for 14 (or up to 30) days. A. Mice were not immunized after P. aquilinum treatment. B. One day after P. aquilinum treatment, mice were immunized with SRBC. C. One day after P. aquilinum treatment, P309 and control mice were sensitized with SRBC and 7 days later were challenged with SRBC. D. One day after P. aquilinum treatment, mice were immunized with TT and 7 days later, splenocytes were challenged with TT in vitro.
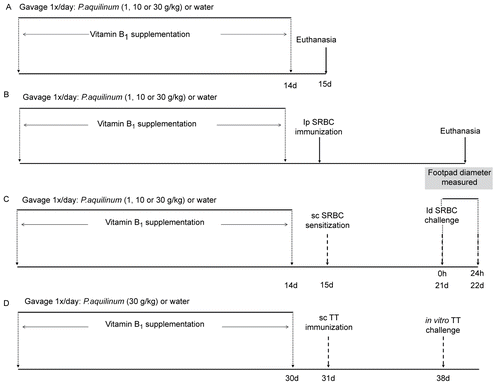
Figure 2. Reduction in the bone marrow cellularity of mice treated with 1 (P1), 10 (P10) and 30 (P30) g/kg BW of P. aquilinum and supplemented with B1 vitamin in water ( 10 mg/ml) for 14 days (p < 0.0001, vs. control values; one-way ANOVA followed by Dunnett’s test). Data are expressed as the mean ± SD (n = 9).
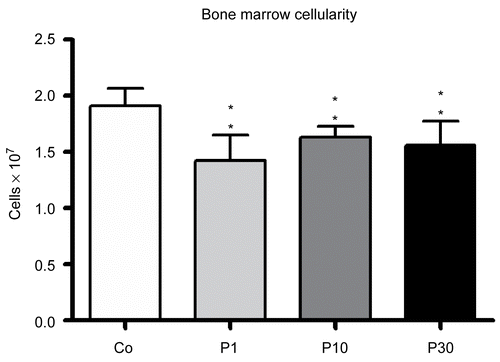
Figure 3. Effects in the spleens of mice treated with 30 (P30) g/kg BW of P. aquilinum and supplemented with B1 vitamin in water ( 10 mg/ml) for 14 days. A. Representative spleen sections from the control group and from the P30 group. Observe smaller white pulp area in spleens from the P30 group. Bar = 100 µm. B. Morphometric analyses showed a significant reduction in white pulp area in spleens from the P30 group (p = 0.004, Mann–Whitney test). Data are expressed as the mean ± SD (n = 9).
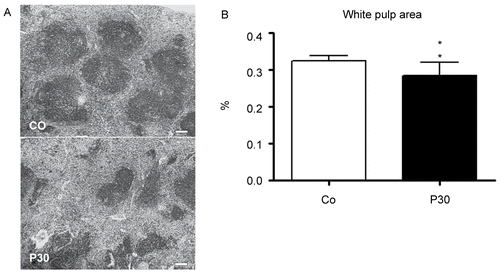
Figure 4. Effects on primary antibody response to SRBC of mice treated with 1 (P1), 10 (P10) and 30 (P30) g/kg BW of P. aquilinum and supplemented with B1 vitamin in water ( 10 mg/ml) for 14 days. The PFC per 105 splenocytes and per spleen and HA titers all remained unaltered in P. aquilinum-treated mice compared with control mice. Data are expressed as the mean ± SD (n = 9).
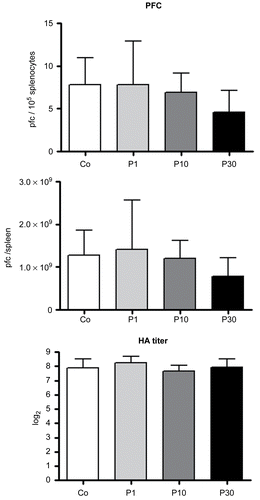
Figure 5. Effects of P. aquilinum treatment on cellular immune response. Measurement of footpad swelling induced by DTH response in mice immunized and challenged with SRBC after treatment with 1 (P1), 10 (P10) or 30 (P30) g/kg BW of P. aquilinum and supplementation with B1 vitamin in water ( 10 mg/ml) for 14 days. P. aquilinum treatment reduced DTH response in the P10 and P30 groups compared with the control group (p = 0.021, one-way ANOVA followed by Dunnett’s test). Data are expressed as the mean ± SD (n = 9).
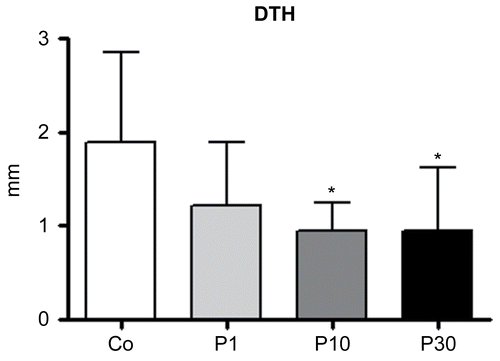
Figure 6. Effects of P. aquilinum treatment on NK cells cytotoxicity. YAC-1 cells were used as a target and effector cells (NK cells) were isolated from the spleens of mice treated with 1 (P1), 10 (P10) or 30 (P30) g/kg BW of P. aquilinum and supplemented with B1 vitamin in water ( 10 mg/ml) for 14 days. P. aquilinum-treatment reduced NK cells cytotoxicity in the P30 group compared with the control group (p = 0.026, Kruskal-Wallis test followed by Dunn’s test). Data are expressed as the mean ± SD (n = 5).
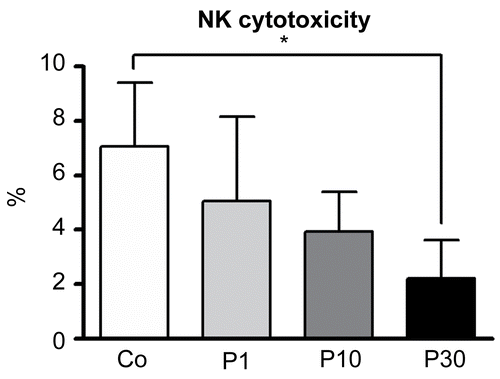
Figure 7. Effects of P. aquilinum treatment on NK cells during TH1 priming. Evaluation of NK cells isolated from the spleens of mice immunized and challenged with TT after treatment with 30 (P30′) g/kg BW of P. aquilinum and supplementation with B1 vitamin in water ( 10 mg/ml) for 30 days. The percentage of total events acquired (20000) are indicated in the graphs. A. P. aquilinum-treatment reduced NK cell numbers (p = 0.015, two-tailed Mann–Whitney test). B. P. aquilinum-treatment diminished IFNγ production by NK cells (p = 0.023, two-tailed Mann–Whitney test). Data are expressed as the mean ± SD (n = 10).
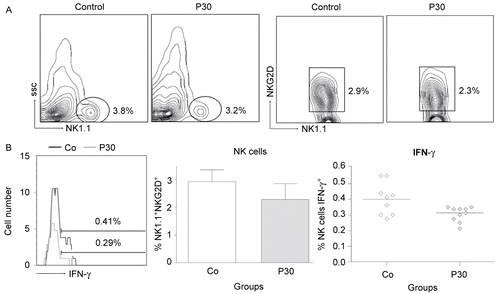
Figure 8. Effects of P. aquilinum treatment on DC cells during TH1 priming. Evaluation of DC cells isolated from the spleens of mice immunized and challenged with TT after treatment with 30 (P30′) g/kg BW of P. aquilinum and supplementation with B1 vitamin in water (10 mg/ml) for 30 days. The percentage of total events acquired (20,000) are indicated in the graphs. A. P. aquilinum-treatment did not alter DC cell maturation. B. P. aquilinum-treatment did not affect IL-12 production by DC cell. Data are expressed as the mean ± SD (n = 10).
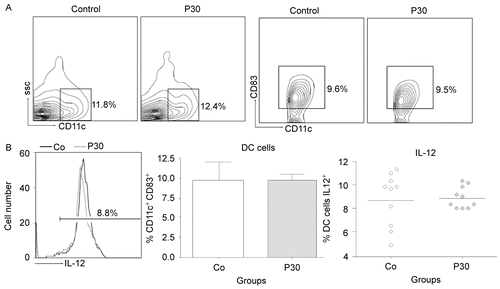
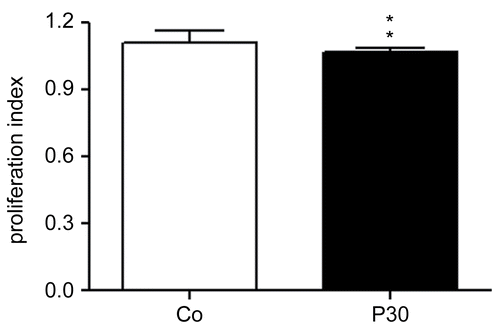
Figure 9. Effects of P. aquilinum treatment on antigen-specific T-lymphocyte expansion. Evaluation of lymphocytes isolated from the spleens of mice immunized and challenged with TT after treatment with 30 (P30′) g/kg BW of P. aquilinum and supplementation with B1 vitamin in water ( 10 mg/ml) for 30 days. P. aquilinum-treatment diminished antigen-specific T-lymphocyte expansion (p = 0.004, two-tailed Mann Whitney test). Data are expressed as the mean ± SD (n = 10).