Figures & data
Figure 1. Deviation in autophagy during initial follicular growth phase: from the origin of oocytes to initial recruitment. Folliculogenesis begins when the precursor primordial germ cells arrive in the ovary. The resultant oogonia increase its population by mitotic division, subsequently induces meiosis. The formation of oocytes is marked with the arrest of meiotic division. Primordial follicle production constitutes the resting pool of follicles. Follicles are continuously recruited from the primordial resting pool to enter the growth phase to become primary follicles. Autophagy supports the growth of oocytes and the formation of the resting follicles pool but opposes the initial recruitment of the primordial follicles. A decrease in the level of autophagy markers (ATG7 and BECN1) directly inhibits oocytes maturation. MTOR inactivation causes an increase in LC3B and BECN1 to enhance autophagy. AMH decreases FOXO3A activation and supports autophagy
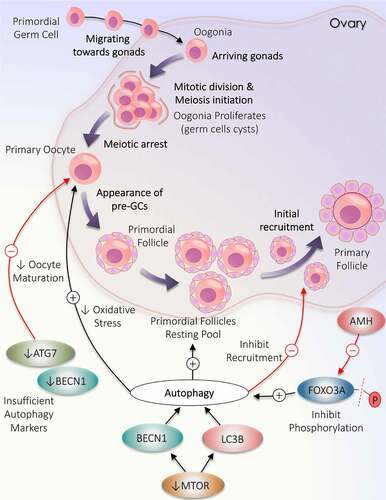
Figure 2. Participation of autophagy in the development of follicles. The developmental process begins with the formation of primordial follicles which is comprising of immature oocyte encircled by a single layer of pre-granulosa cells. The primary follicle is defined by granulosa cells with the appearance of zona pellucida. The proliferation of granulosa cells to multiple layers and the formation of theca cells layer results in the development of secondary follicle. Follicle with multiple small cavities is referred to as early antral follicles. A follicle with a single large cavity filled with antral fluid is called the antral follicle. Cyclic recruitment of antral follicles leads to the formation of a fully matured Graafian follicle. Estradiol and INH (inhibin) are secreted by the Graafian follicle, which stops the recruitment process. Activation of AKT and MTOR in granulosa cells prevents autophagy and favors follicular development. After the selection of dominant follicles, the remaining subordinate follicles are degenerated by autophagy-induced apoptosis. Increased expression of OLR1 receptor in granulosa cells of regressing follicles supports autophagy via inducing ROS production, resulting in granulosa cells death by apoptosis. NOX: NADPH oxidase
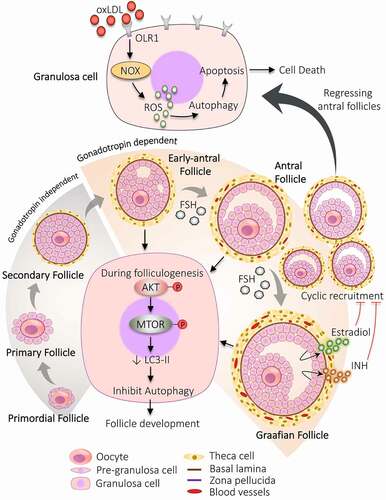
Figure 3. Autophagy implication during ovulatory and post-ovulatory events. Autophagy regulates the functioning of follicular cells during and after ovulation. (A) Ovulation: Disturbed level of autophagy markers in cumulus cells attached with oocytes damages the oocyte, which hampers the oocyte quality resulting in infertility. (B) Corpus luteum: becn1−/- causes a defect in lipid droplet formation and affects steroidogenesis decreases progesterone production. (C) Regression of corpus luteum: Enhanced expression of autophagy markers along with autophagic gene expression, inhibition of MTOR pathway, and activation of MAPK1-MAPK3 pathway triggers the autophagy and autophagy induced apoptosis
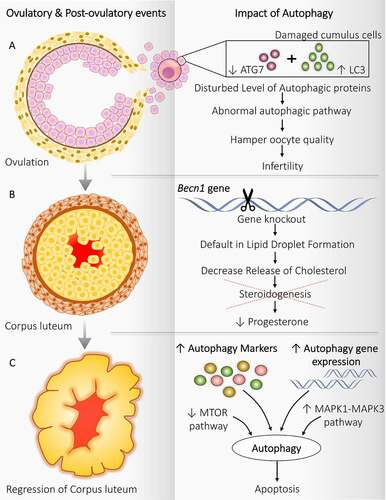
Figure 4. Significance of autophagy during the follicular atresia. Atresia is noticed during all the developmental stages of follicles; however, maximum atresia is observed at the antral stage. Autophagy contributes to follicular atresia by induction of apoptosis and/or autophagic cell death of granulosa cells. Activation of MAPK8 triggers autophagy by phosphorylation of apoptotic protein BCL2. Phosphorylated BCL2 is detached from the autophagy protein BECN1 and promotes autophagy. FSH hormone prevents oxidative stress damage by inhibiting autophagy via PI3K-AKT-MTOR pathway activation and mitophagy
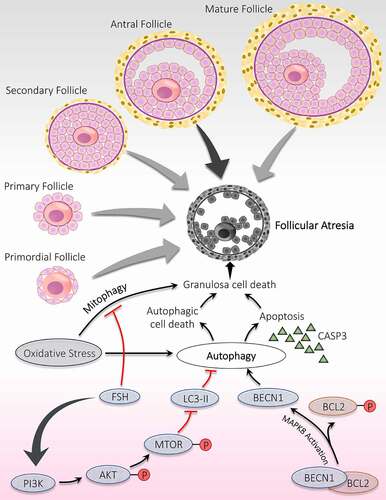
Figure 5. Link between autophagy and hyperandrogenism in PCOS. Different autophagic responses are observed at various locations due to an increased level of androgen in PCOS. (A) Theca cells: Increase in palmitic acid augments SQSTM1 and ubiquitin, which inhibits autophagy. Declined autophagy induces testosterone production by increasing ROS and enhanced mRNA expression of CYP17A1 and SERPINE1 via MAPK/p38 and MAPK8 activations. (B) Skeletal Muscles: Testosterone causes activation of MTORC1, which inhibits the autophagy proteins and suppresses autophagy. (C) Uterus: Altered gene expression in response to hyperandrogenemia inhibits autophagy. (D) Granulosa Cells: Testosterone induces expression of BECN1, ATG, LC3, and favors autophagy. Red arrow: deactivation; Black arrow: activation
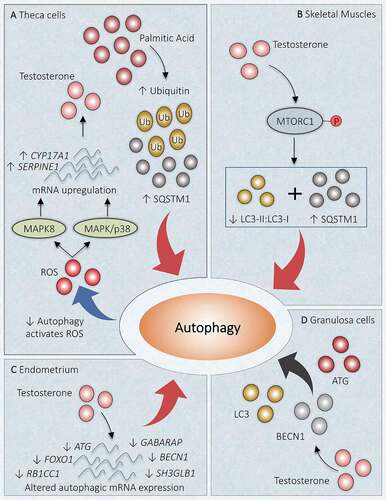
Figure 6. Autophagy adaptations during anovulation associated with PCOS. An altered population of follicles at the initial stages of follicular development including, an increase in the number of primary follicles and pre-antral follicles. Autophagy causes granulosa cell death in the pre-antral follicle and leads to follicular atresia. Heightened mitophagy in the cumulus cells in response to oxidative stress in follicular fluid. Defective mitophagy causes oocyte damage causes deformation of oocytes. Autophagy prevents the transition of antral follicles to the pre-ovulatory stage and opposes ovulation. An increase in the level of INS, LH, and testosterone prevents ovulation by augmenting the cAMP level
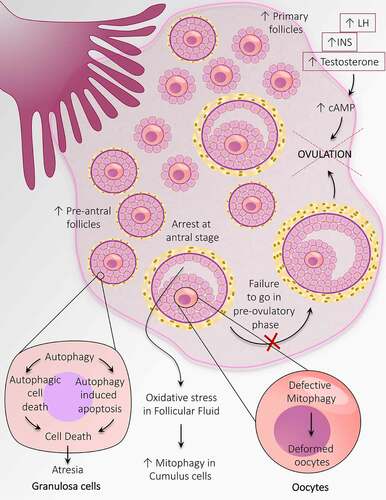
Figure 7. Disturbed folliculogenesis in PCOS and its connection with autophagy. Different autophagic pathways are involved in altered folliculogenesis in the PCOS ovary. An increase in ROS promotes autophagy and the formation of cysts. An increase in TUG1 expression suppresses autophagy by obstructing the MAPK/ERK pathway and supports antral follicle survival. Activation of the MTOR pathway inhibits autophagy and promotes aberrant growth of follicles
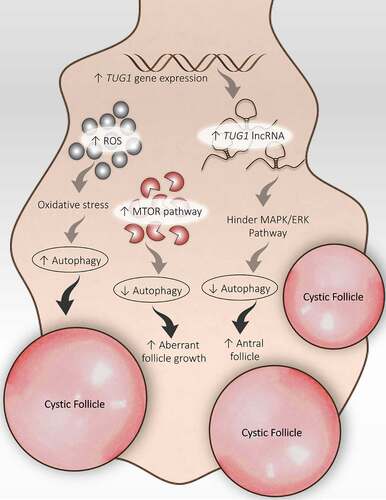
Figure 8. Autophagy role in metabolic abnormalities acquired during PCOS in various metabolic organs. Autophagy plays a crucial part in governing the metabolic response in affected tissues in PCOS. (A) Adipose tissue: Obesity-driven INS resistance because of adipocyte dysfunction and defaulted INS signaling pathway is suppressed via the induction of autophagy. (B) Ovary: Hyperinsulinemia and hyperandrogenism both share a direct relationship, where INS promotes the expression of steroidogenic enzymes ARK1C3 and CYP17A1. Regulation of autophagy inhibits INS resistance and hyperandrogenemia. (C) Skeletal muscles: Autophagy is attenuated in PCOS via activation of MTOR accounts for INS resistance. (D) Heart: Cardiac autophagy gets halted by decreased autophagy protein level and decreased AMPK phosphorylation responsible for causing hypertrophy in cardiac cells, leading to cardiovascular (CVS) complications. (E) Liver: Decline in turnover of autophagosomes is observed in response to decreased vitamin D level, contributes to liver disease which gets suppressed on autophagy stimulation
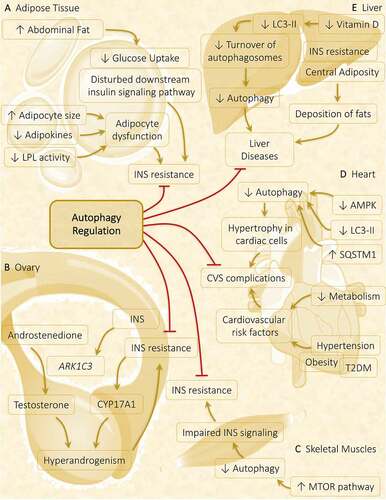
Figure 9. Mechanisms governing autophagy during different conditions related to PCOS. In PCOS, autophagy is depicted in response to different conditions such as (A) Nutrient deprivation: In ovaries, phosphorylation of AMPK induces deacetylation of LC3 via SIRT1 activation. Activated LC3 binds with TP53INP2, which promotes its translocation from the nucleus to the cytoplasm, where it can bind with ATG7 and initiates autophagosome formation. (B) Inflammation: Autophagy activation is accompanied by inflammation in PCOS via an increase in HMGB1, which stimulates autophagy in granulosa cells and causes its death. Whereas, an enhanced level of IGF1 is involved in governing autophagy via the PI3K-AKT-FOXO1-MTOR pathway. (C) Endometrial hyperplasia: Defaulted autophagy triggers hyperplasia in the endometrium in PCOS, which is reduced by induction of autophagy and autophagy induced apoptosis
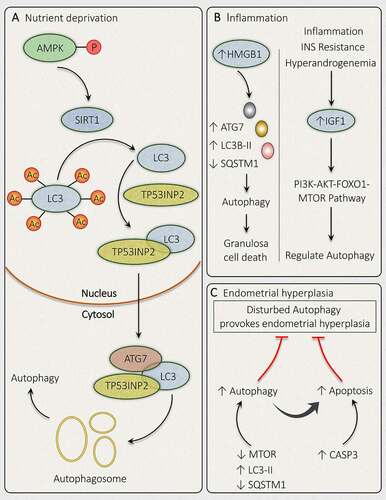
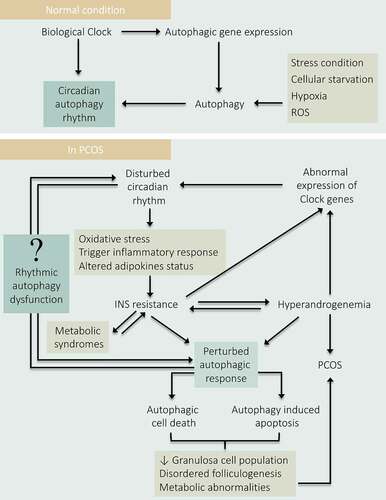
Figure 10. Depicting the role of Circadian-autophagy-rhythm in PCOS. Under normal physiology, the circadian biology of the body regulates autophagy by governing the rhythmic expression of the autophagic genes. Conversely, autophagy also governs the circadian rhythm. Therefore, a balance between autophagy and circadian biology is necessary for normal physiology. In PCOS: Disturbance in circadian rhythm affects the normal physiology of the body and resulted in INS resistance which causes hyperandrogenemia, metabolic abnormalities, and damages autophagic responses leading to PCOS pathogenesis. Where hyperandrogenemia and IR affect circadian biology by hindering the expression of clock genes. Altogether, states a direct link between circadian arrhythmicity and damaged autophagy in PCOS progression