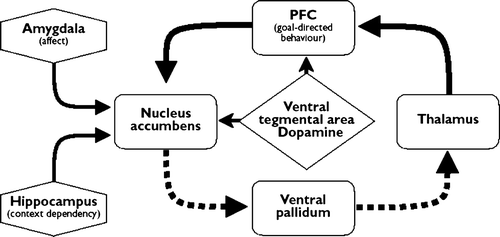
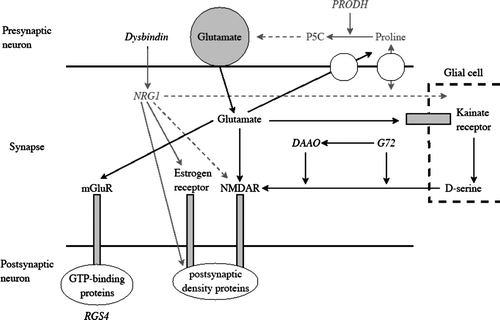
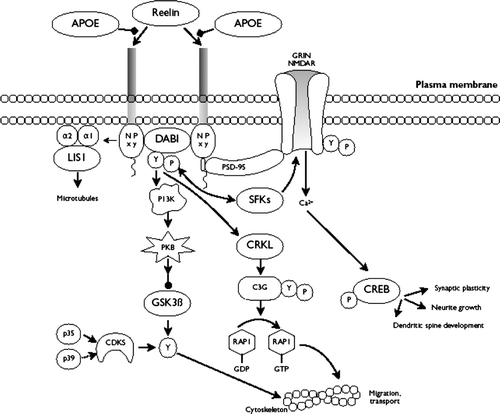
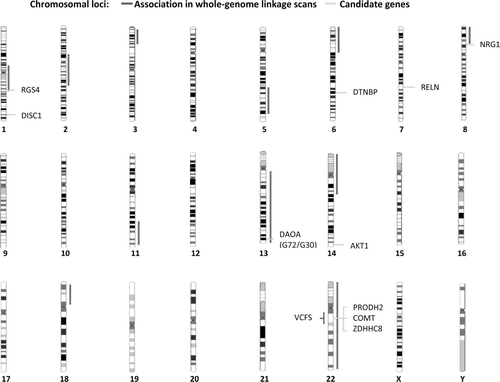
Figures & data
Table I. Imaging studies of striatal presynaptic dopamine parameters in drug-naive (DN), drug free (DF) and treated (T) patients with schizophrenia.
Table II. Glutamate parameters measured in brain tissue of schizophrenia patients
Table III. Synopsis of selected characteristics of candidate endophenotypes in schizophrenia research (pers. comm. A. Jablensky)
Table IV. CSF proteins and lipids involved in the pathophysiology of schizophrenia. Several CSF proteins and lipids are important in transport, nerve cell excitability, nerve cell growth and neuron secretion. These functions of lipids may have significant physiologic ramifications in schizophrenia (pers. comm. A. Fonteh)
Table V. Candidate genes for schizophrenic psychoses and strength of the biological evidence