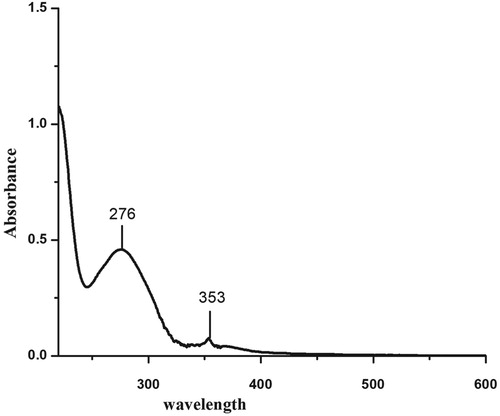
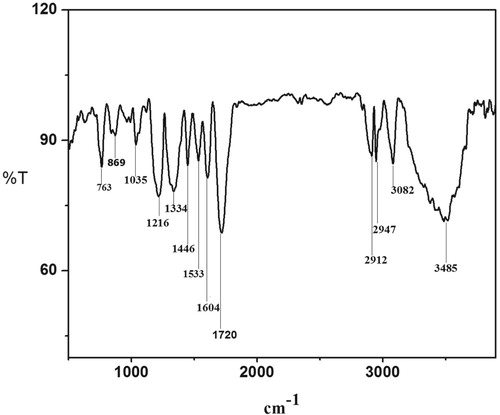
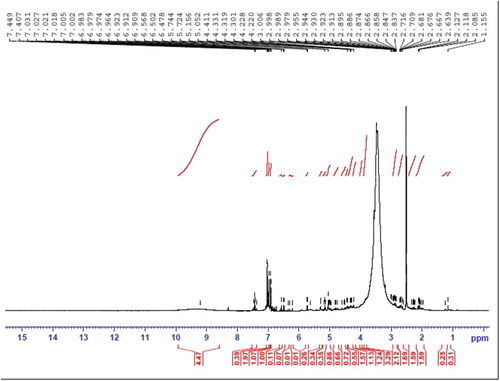
Figures & data
Table 1. The free-radical scavenging activity of different fractions of T. chebula fruit and ascorbic acid (positive control).
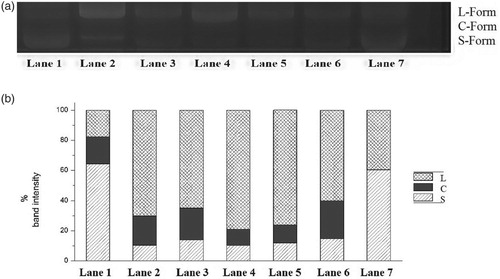
Figure 1. (a) Inhibitory effect of T. chebula fractions on plasmid DNA nicking. Lane 1 – DNA (pEGFP-C1 plasmid alone); Lane 2 – DNA and Fenton reagent; Lane 3 – DNA, Fenton reagent and Chloroform–Acetone 1:4; Lane 4 – DNA, Fenton reagent and Ethyl acetate–Ethanol 6:1; Lane 5 – DNA, Fenton reagent and Hexane–Ether 1:4; Lane 6 – DNA, Fenton reagent and Ether–Acetone 1:1; Lane 7-DNA, Fenton reagent and Methanol–Water 1:1. (b) Band intensity of Circular, Linear and Relaxed forms. Lane 1 – DNA (pEGFP-C1 plasmid alone); Lane 2 – DNA and Fenton reagent; Lane 3 – DNA, Fenton reagent and Chloroform–Acetone 1:4; Lane 4 – DNA, Fenton reagent and Ethyl acetate–Ethanol 6:1; Lane 5 – DNA, Fenton reagent and Hexane–Ether 1:4; Lane 6 – DNA, Fenton reagent and Ether–Acetone 1:1; Lane 7-DNA, Fenton reagent and Methanol–Water 1:1.