Figures & data
Table 1. Product and formulation characteristics of the 5-grass pollen and 1-grass pollen SLIT tablets
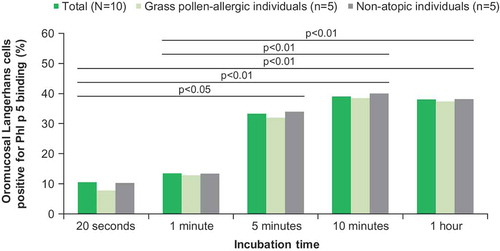
Figure 1. Kinetics of allergen activity release and dissolution of 300 IR and 100 IR HDM and 5 grass SLIT tablets in vitro.
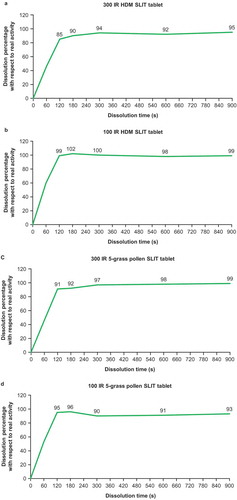
Figure 2. Kinetics of grass pollen allergen binding by oromucosal Langerhans cells ex vivo.