Figures & data
Figure 1. At the first surgery, the revision protocol was initiated to engender a tissue reaction similar to that surrounding an aseptically loosened implant. Specifically, a 6.0-mm plasma-sprayed titanium implant axially pistoned 0.5 mm concentrically in a hole with a 0.75-mm gap. The implant pistons inward by engagement of the PE endcap with the tibial plateau. Following the loading episode, the internal spring pushes the implant outwards to move it back to its original position. The implants were weight-bearing in each medial femoral condyle for 8 weeks in the presence of PE particles (0.5–50 μm; 0.5 × 108particles; 85% < 12 μm). Following insertion of the implant, the surgical site was not lavaged further nor irrigated. At the second surgery 8 weeks later, the right-side implant was modified to prevent it from pistoning (i.e. the PE end plug was cut to prevent engagement with the tibia on the opposite side). The left side implant continued to piston after it was observed in a sham operation.
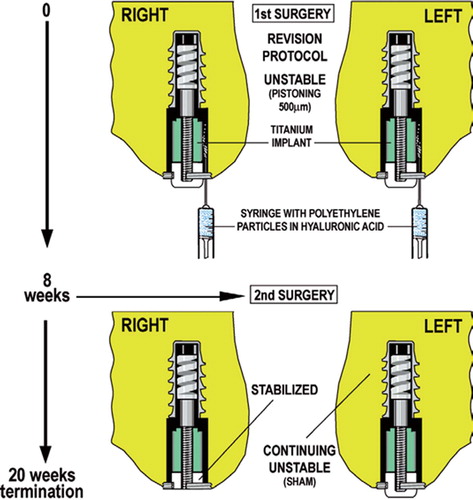
Figure 2. Implant shear pushout test. Bars represent stable and unstable implants with and without the bisphosphonate alendronate (+ BP and – BP).
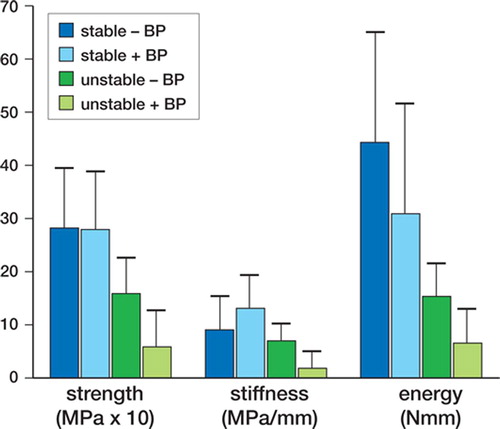
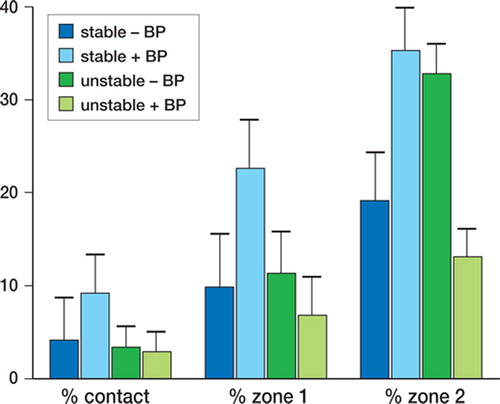
Figure 3. Mean and standard error for percentage of bone in contact with implant (bone ongrowth), percentage of bone in zone 1 (half of gap closest to the implant surface) and percentage of bone in zone 2 (half of gap closest to the original drill hole), for stable and unstable implants with and without the bisphosphonate alendronate (+ BP and – BP).