Figures & data
Table 1. Definitions of histologic disease activity targets in ulcerative colitis.
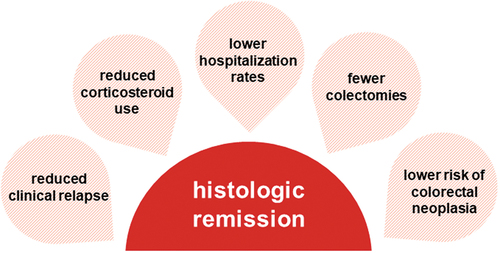
Figure 1. UC treatment targets.
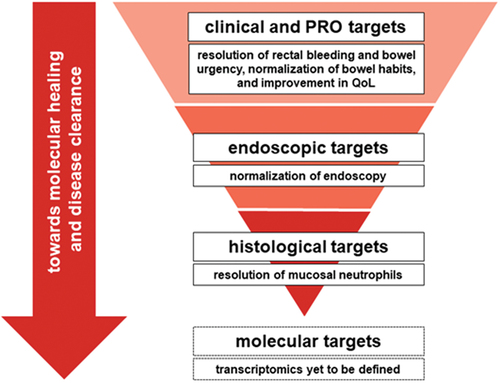
Figure 2. Timeline of selected UC histology assessments and regulatory guidance.
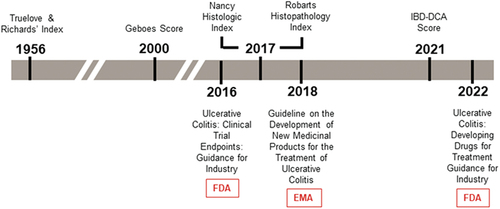
Table 2. Scoring of selected histologic assessments.
Table 3. Histologic disease activity endpoints in clinical trials of treatments for UC.
Table 4. Selected studies analyzing associations between histologic remission and clinical outcomes.