Figures & data
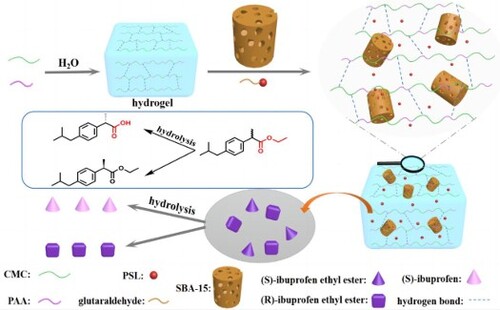
Figure 1. Schematic illustration of CMC-g-PAA/SBA-15 composite hydrogels formation and immobilization PSL enantioselective hydrolysis racemic ibuprofen ethyl ester mechanism.
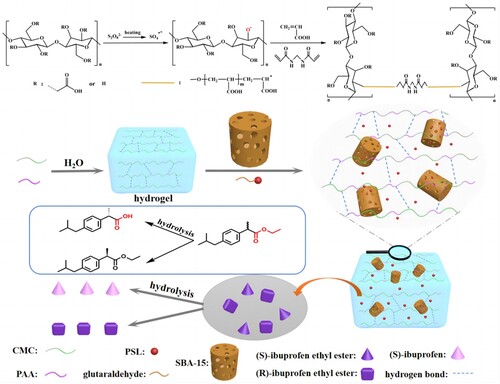
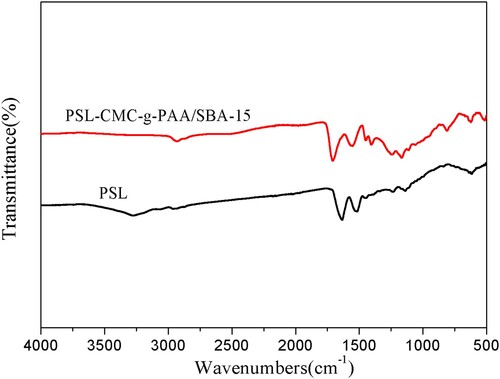
Figure 2. The FT-IR spectra of (a) SBA-15, (b) Cellulose extracted from corn stalks, (c) CMC, (d) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.6 wt% MBA), (e) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.5 wt% MBA) and (f) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.4 wt% MBA)
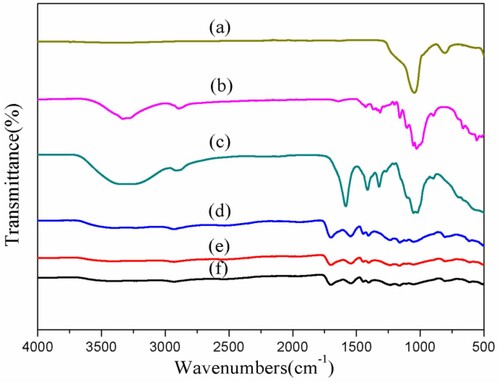
Figure 3. SEM images of (a) SBA-15, (b) Cellulose extracted from corn stalks, (c) Carboxymethyl cellulose, (d) CMC-g-PAA, (e) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.5 wt% MBA), (f) CMC-g-PAA/SBA-15 (12 wt% CMC, 0.6 wt% SBA-15, and 0.6 wt% MBA) and (g) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.4 wt% MBA).
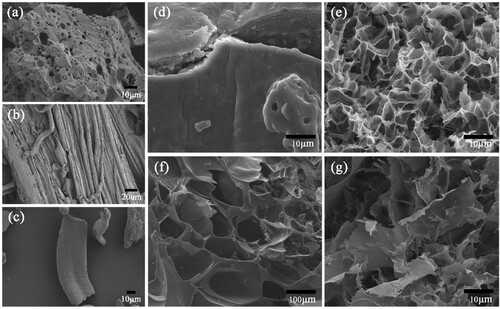
Figure 4. X-ray diffraction patterns of (a) Cellulose extracted from corn stalks, (b) CMC, (c) SBA-15, (d) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.6 wt% MBA), (e) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.5 wt% MBA) and (f) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.4 wt% MBA)
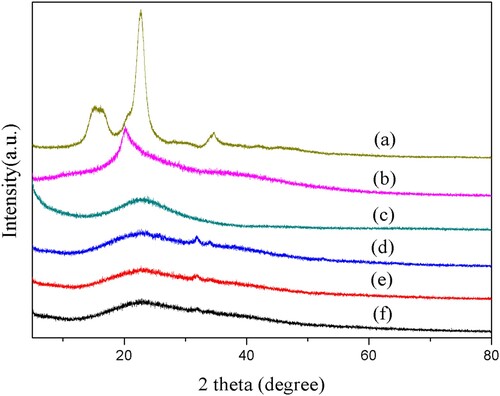
Figure 5. Thermal characteristics of (a) Cellulose extracted from corn stalks, (b) CMC, (c) CMC-g-PAA/SBA-15(12 wt% CMC, 0.6 wt% SBA-15, and 0.6 wt% MBA) and (d) CMC-g-PAA/SBA-15(10 wt% CMC, 0.6 wt% SBA-15, and 0.4 wt% MBA)
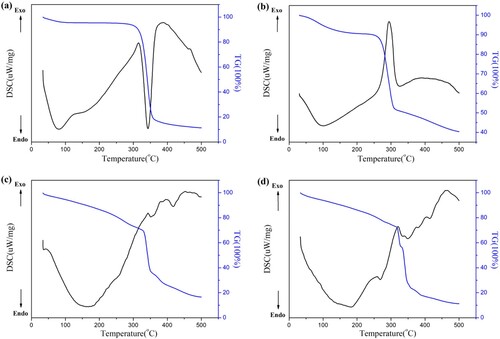
Table 1. Comparison of the catalytic properties of free and immobilized PSL.
Figure 7. Effect of temperature on free (a) and immobilized (b) the enzymatic hydrolysis of ibuprofen ethyl ester. The standard reaction was carried out in a 20 ml round-bottom flask containing free PSL 13 mg or immobilized PSL 40 mg (protein content: 13 mg), 1 mmol racemic ibuprofen ethyl ester, and 5 mL of 50 mM sodium acetate buffer (pH 7.0). The resulting mixture was shaken at different temperature (20-60°C) (180 rpm).
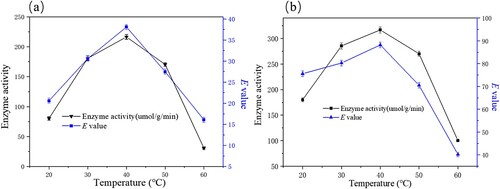
Figure 8. Effects of pH on the free (a) and immobilized (b) enzymatic hydrolysis of ibuprofen ethyl ester. The standard reaction was carried out in a 20 ml round-bottom flask containing free PSL 13 mg or immobilized PSL 40 mg (protein content: 13 mg), 1 mmol racemic ibuprofen ethyl ester, and 5 mL of 50 mM sodium acetate buffer (pH 5.0-8.0). The resulting mixture was shaken at 40°C (180 rpm).
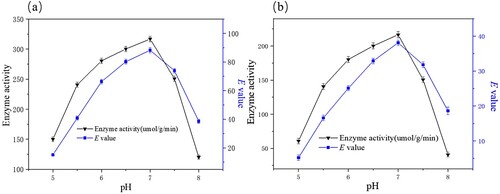
Figure 9. Effect of time on the free (a) and immobilized (b) enzymatic hydrolysis of ibuprofen ethyl ester. The standard reaction was carried out in a 20 ml round-bottom flask containing free PSL 13 mg or immobilized PSL 40 mg (protein content: 13 mg), 1 mmol racemic ibuprofen ethyl ester, and 5 mL of 50 mM sodium acetate buffer (pH 7.0). The resulting mixture was shaken at 40°C (180 rpm) for (0-4 h).
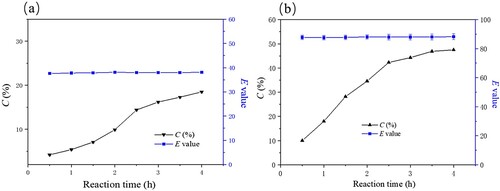
Figure 10. Reusability of immobilized PSL on the enzymatic hydrolysis of ibuprofen ethyl ester. The standard reaction was carried out in a 20 ml round-bottom flask containing 40 mg immobilized PSL, 1 mmol racemic ibuprofen ethyl ester, and 5 mL of 50 mM sodium acetate buffer (pH 7.0). The resulting mixture was shaken at 40°C (180 rpm).
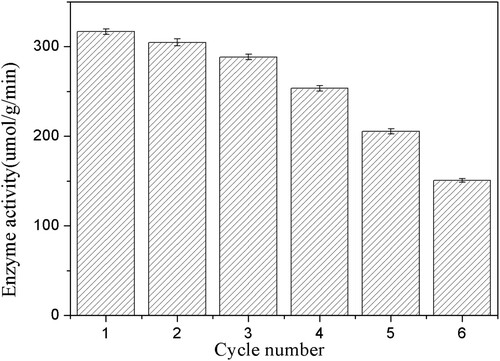
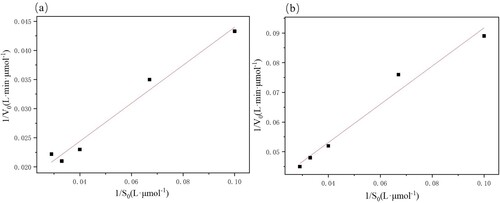
Table 2. Parameters obtained from fitting the Lineweaver- burk polt.